Authors: Jared Spilka, MD and Michael A. Evans, MD – Ann & Robert H. Lurie Children’s Hospital of Chicago, Northwestern Feinberg School of Medicine
A 3-month-old presents to the cardiac catheterization laboratory for hemodynamic measurements and coronary angiography. Based on the angiograms illustrated below, what is the MOST LIKELY diagnosis (Image source, authors)?
The Correct Answer is C. Ventriculo-coronary fistulae
Pulmonary atresia with intact ventricular septum (PA/IVS) is a congenital heart defect that occurs in about 0.6/10,000 live births. PA/IVS represents a large spectrum of anatomic variety. This anatomy varies with respect to the tricuspid valve development, degree of RV hypoplasia, infundibular development, and presence of coronary stenosis and/or ventriculo-coronary fistulae. The development of the right ventricle and pulmonary valve ranges from membranous atresia of the pulmonary valve with mildly hypoplastic right ventricle (RV) and tricuspid valve (TV) and a well-developed infundibulum to a severely hypoplastic RV and TV, an underdeveloped infundibulum, and right-ventricular-dependent coronary circulation (RVDCC). As a result, the patent ductus arteriosus (PDA) is the sole source of pulmonary blood to the pulmonary arteries, which are typically confluent. Thus, PA/IVS is considered single ventricle physiology at birth. An obligate right-to-left atrial level shunt allows for survival in-utero and after delivery. In addition, the tricuspid valve is abnormal, ranging from hypoplastic and severely stenotic to severely regurgitant. In some cases, the tricuspid valve is described as Ebsteinoid in character.
A small tricuspid valve with a low Z-score and severe stenosis is associated with a severely hypertensive RV in which there is no egress of blood via the right ventricular outflow tract (RVOT). As a result, embryological coronary sinusoids act as a means of egress of blood while in utero and likely lead to the development of RV to coronary artery fistulae. The turbulent flow within these fistulae likely contribute to stenosis of the coronary arteries and at times interruptions of normal aorto-coronary blood flow leading to right ventricle dependent coronary circulation (RVDCC). In this circumstance, attempts to open the RVOT and decompress the right ventricle will lead to decreased perfusion of the ventricular wall, myocardial ischemia, and probable mortality. Hemodynamic cardiac catheterization with angiography is therefore critical to rule out the presence of RVDCC prior to any catheter-based or surgical intervention.
The demonstrated angiographic video shows the following:
• Right ventricular hand injection (upper right and left videos)
o Hypoplastic, muscle-bound RV
o Lack of blood flow through the pulmonary valve
o Multiple ventriculo-coronary fistulae to the proximal right main coronary artery and mid to distal left anterior descending (LAD)
o A ventriculo-coronary fistulous connection to a large collateral traveling leftward and filling the distal LAD
• Selective left coronary angiogram (lower right and left videos)
o Left circumflex (LCx), left obtuse/marginal (OM), and sinoatrial nodal branches seen in their entirety
o Truncated view of proximal LAD
o Lack of visualization of the mid to distal LAD
o Complete wash-in (reversal of flow) of the LAD/diagonal and left main coronary artery
It can be deduced that this patient does not have RVDCC. In terms of the left coronary artery, there is dual blood supply from the RV through coronary fistulous connections and antegrade flow to the proximal LAD and LCx. The selective left coronary angiograms do not demonstrate coronary ostial atresia. However, a right selective coronary angiogram or an aortogram would be needed to demonstrate the patency of the right coronary ostia. Ultimately, this patient falls in a grey zone and could tolerate RV decompression.
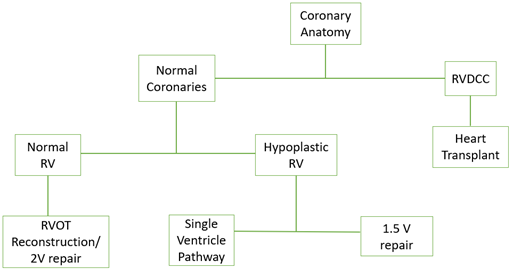
In the presence of RVDCC and coronary ostial atresia/stenoses, patients often require transplant evaluation and a systemic to pulmonary artery surgical shunt or PDA stent to maintain pulmonary blood flow and pressurization of the RV as a bridge. In some cases of PA/IVS, the single ventricle pathway culminating in the Fontan palliation may be undertaken, but all stages tend to have significant risk of morbidity and mortality. In less-severe phenotypes of PA/IVS, without the presence of RVDCC for example, it is reasonable to plan for a repair involving biventricular circulation. A simplified management algorithm can be seen below.
Anomalous left (or right) coronary artery from the pulmonary artery (ALCAPA/ARCAPA) is a defect in which one of the coronary arteries arises from the pulmonary artery resulting in myocardial perfusion with significantly desaturated blood. This eventually leads to myocardial ischemia and cardiomyopathy and requires surgical correction. Contrast injected into the pulmonary artery would reveal flow into the associated coronary artery in ALCAPA and ARCAPA.
Single coronary artery (SCA) is a defect in which the myocardium is perfused with a coronary artery that arises from a single coronary ostium. A left coronary artery may arise from the single right coronary or vice versa. This coronary pattern is noted with frequency in D-transposition of the great arteries, requires careful evaluation during repair, and can affect outcomes in D-TGA. Aortic root angiography would demonstrate contrast flow into a single ostium that then branches into right and left coronaries.
Cardiac allograft vasculopathy (CAV) is an accelerated form of coronary artery disease (CAD) that occurs due to concentric intimal hyperplasia (thickening) along the entire course of a coronary vessel. This pathology occurs after orthotopic heart transplantation and contributes significantly to mortality. The treatment for CAV is retransplantation. CAV is challenging to diagnose, as angiography is not as sensitive for the detection diffuse areas of narrowing that are seen in CAV. Focal areas of narrowing and plaques as seen in CAD are more easily detected by angiography. Many other imaging modalities are utilized to attempt early detection of CAV, which include vascular ultrasound, optical coherence tomography, computed tomography angiography, and cardiac MRI.
REFERENCES:
Ungerleider R.M. et al. Critical Heart Disease in Infants and Children. 3rd Edition. Elsevier. 2019.
Chikkabyrappa SM, Loomba RS, Tretter JT. Pulmonary Atresia With an Intact Ventricular Septum: Preoperative Physiology, Imaging, and Management. Semin Cardiothorac Vasc Anesth. 2018;22(3):245-255. doi:10.1177/1089253218756757
Desmet W, Vanhaecke J, Vrolix M, et al. Isolated single coronary artery: a review of 50,000 consecutive coronary angiographies. Eur Heart J. 1992;13(12):1637-1640. doi:10.1093/oxfordjournals.eurheartj.a060117
Pober JS, Chih S, Kobashigawa J, Madsen JC, Tellides G. Cardiac allograft vasculopathy: current review and future research directions. Cardiovasc Res. 2021;117(13):2624-2638. doi:10.1093/cvr/cvab259
Rickenbacher PR, Pinto FJ, Chenzbraun A, et al. Incidence and severity of transplant coronary artery disease early and up to 15 years after transplantation as detected by intravascular ultrasound. J Am Coll Cardiol. 1995;25(1):171-177. doi:10.1016/0735-1097(94)00323-i
Ramzy D, Rao V, Brahm J, Miriuka S, Delgado D, Ross HJ. Cardiac allograft vasculopathy: a review. Can J Surg. 2005;48(4):319-327.
