Authors: JE Elliott, MD, Dept of Anesthesiology, Critical Care and Pain Medicine, University of Texas Health Science Center at Houston/McGovern Medical School, Houston, TX AND
M Gangadharan, MBBS, FAAP, FASA, Dept of Anesthesiology, Critical Care and Pain Medicine, University of Texas Health Science Center at Houston/McGovern Medical School, Houston, TX
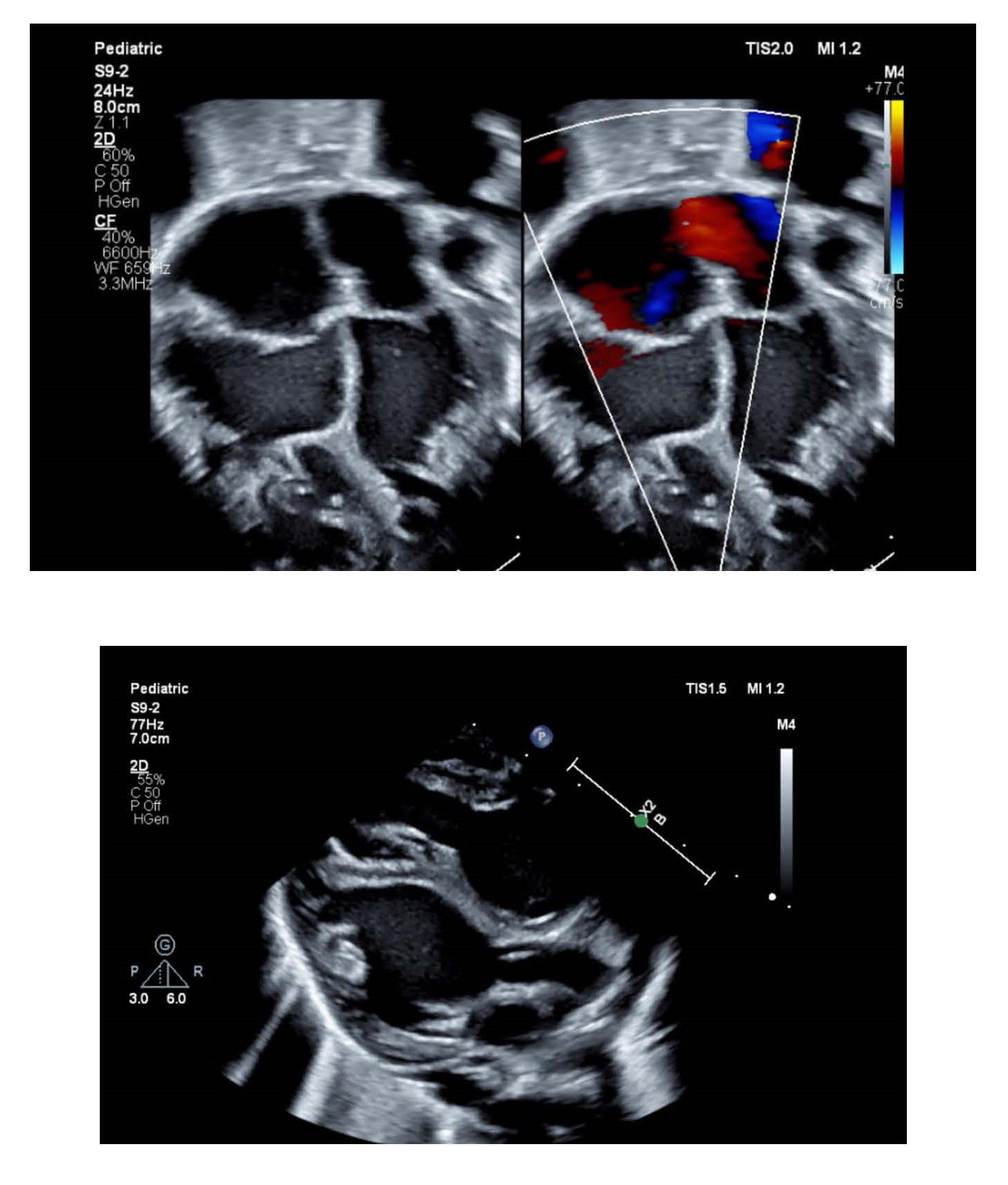
A three-day-old boy with significant cardiomegaly has a transthoracic echocardiogram that demonstrates a globular appearing left ventricle with severely depressed systolic function. Several transthoracic echocardiographic images are illustrated below. Which of the following types of cardiomyopathies is MOST likely present in this patient?
EXPLANATION
Non-compaction cardiomyopathy was considered a rare condition but is being increasingly diagnosed because of heightened awareness. The left ventricular myocardium of patients with non-compaction cardiomyopathy is characterized by prominent trabeculations and deep intertrabecular recesses which communicate with the ventricular cavity. Non-compaction cardiomyopathy results from a defect in normal myocardial development. The primitive myocardium is a trabecular network of sponge-like muscle fibers in the mid-late embryo, and receives its blood supply from intertrabecular spaces that communicate with the cardiac chambers. During normal development, these trabeculations disappear after epicardial coronary arterial supply has been established. Subsequently, the spongy myocardium is transformed into the compact normal myocardium. Non-compaction results when this transformation from spongy to compact myocardium fails to occur, resulting in an inner prominent spongy layer with a thin outer compact epicardial layer.
Clinically, non-compaction cardiomyopathy is characterized by heart failure, arrhythmias and thromboembolic events that may present at any age. There are several subtypes of left ventricular noncompaction cardiomyopathy (LVNC) and these different phenotypes have prognostic implications. The subtypes are: (1) Isolated non-compaction with normal cardiac function; (2) non-compaction with hypertrophic cardiomyopathy (HCM), and, (3) Non-compaction with dilated cardiomyopathy (DCM). A 2015 study by Jeffries et al, based on the Pediatric Cardiomyopathy Registry, investigated time to death or transplantation in patients with different phenotypes of LVNC. They observed that children with isolated LVNC had the best outcomes followed by LVNC with HCM. LVNC with DCM resulted in the worst patient outcomes. LVNC may be also associated with a variety of congenital heart defects including anomalous coronary arteries, atrial and ventricular septal defects, Ebstein’s anomaly, transposition of the great arteries, absent pulmonary valve, pulmonary stenosis, hypoplastic left heart syndrome, and anomalous pulmonary veins. Reversible forms of LVNC have been reported to occur when the left/systemic ventricle is subjected to abnormal loading conditions such as during pregnancy and remodeling of the right ventricle in corrected transposition of the great vessels.
Although echocardiography is the most common modality used to confirm diagnosis, there are not widely accepted criteria that constitute a gold standard for the diagnosis of LVNC. However, there is general agreement that LVNC is defined by presence of the following characteristics: (1) prominent trabeculations in the left ventricle; (2) deep recesses between the trabeculations and the presence of a thin compacted layer; and (3) a ratio > 2:1 of the noncompacted to compacted myocardial layers at end-systole. Cardiac magnetic resonance (CMR) is also being increasingly utilized to delineate the non-compacted muscle layer and to better visualize the cardiac apex, in addition to measuring ejection fraction. Cardiac fibrosis can also be evaluated with the use of late gadolinium enhancement during CMR.
Management should follow evidence-based guidelines for the specific phenotype of LVNC. Heart failure is managed according to standard guidelines. These patients are at increased risk for thromboembolic events such as stroke and may require anticoagulation. Typically, most patients are started on oral aspirin therapy but may require additional medications such as warfarin if there are other risk factors such as reduced ejection fraction or atrial fibrillation. Ventricular arrhythmias are common, and these patients may need placement of a defibrillator to prevent sudden death. First-degree relatives should be screened as one study demonstrated that 30% of the relatives screened by echocardiography were found to have LVNC.
Neonatal non-compaction cardiomyopathy appears to be associated with particularly poor outcomes. In a small retrospective analysis by Rodrigues-Fanjul et al, the mortality rate of 14 neonates with non-compaction cardiomyopathy was 42.8% (6) over a median follow-up period of 34 months. Five of the six deaths were from ventricular failure. The presence of biventricular involvement and poor ventricular function were associated with a higher risk of death.
Echocardiographic features of hypertrophic cardiomyopathy (HCM) include wall thickness > 15mm, asymmetrical hypertrophy, systolic anterior motion of the mitral valve (SAM), and a small LV cavity. Echocardiographic features of dilated cardiomyopathy (DCM) include left ventricular (LV) dilation (>112%, corrected for age and body surface area) and systolic dysfunction (LV ejection fraction < 45%) with impaired global contractility (fractional shortening < 25%). Although subsets of noncompaction cardiomyopathy include dilated and hypertrophic forms, the presence of trabeculations in both ventricles make biventricular noncompaction cardiomyopathy the most likely diagnosis in the patient presented in the stem.
REFERENCES
Ichida F. Left ventricular noncompaction - Risk stratification and genetic consideration. J Cardiol. 2020;75(1):1-9. doi:10.1016/j.jjcc.2019.09.011
Jeffries JJ, Wilkinson JD, Sleeper LA et al. Cardiomyopathy phenotypes and outcomes for children with left ventricular myocardial noncompaction: Results from the Pediatric Cardiomyopathy Registry. J Cardiac Fail. 2015; 21:877-884.
CE Bennett and R Freudenberger. The Current Approach to Diagnosis and Management of Left Ventricular Noncompaction Cardiomyopathy: Review of the Literature. Cardiology Research and Practice. Volume 2016, Article ID 5172308, 1-7.
J Rodriguez-Fanjul, S Tubio-Comez, JMC Bellon, C Bautista-Rodriguez, and J Sanchez-de-Toledo. Neonatal Non-compacted Cardiomyopathy: Predictors of Poor Outcome. Pediatric Cardiology. 2020; 41:175-180.
Maron BJ, Desai MY, Nishimura RA, et al. Diagnosis and Evaluation of Hypertrophic Cardiomyopathy: JACC State-of-the-Art Review. J Am Coll Cardiol 2022;79(4):372-389. doi:10.1016/j.jacc.2021.12.002
Mathew T, Williams L, Navaratnam G, et al. Diagnosis and assessment of dilated cardiomyopathy: a guideline protocol from the British Society of Echocardiography. Echo Res Pract. 2017;4(2):G1-G13. doi:10.1530/ERP-16-0037
