Authors: Amy Babb, MD - Vanderbilt University and Kaitlin M. Flannery, MD, MPH - Stanford University
A 16-year-old girl with a history of orthotopic heart transplantation is admitted with one week of nausea, vomiting, and fatigue. She is scheduled for an urgent endomyocardial biopsy due to suspected rejection. Past medical history includes stage 3 chronic kidney disease and type 2 diabetes, and current medications are aspirin, atorvastatin, carvedilol and empagliflozin. Which of the following laboratory values is MOST likely to be associated with euglycemic diabetic ketoacidosis associated with empagliflozin?
EXPLANATION
Empagliflozin, dapagliflozin, canagliflozin, and ertugliflozin are sodium-glucose cotransporter-2 (SGLT2) inhibitors used for managing type 2 diabetes, chronic kidney disease, and heart failure. SGLT2 inhibitors work by blocking reabsorption of glucose and sodium in the proximal renal tubules leading to glucosuria, natriuresis, and diuresis. Adult studies and limited pediatric studies have shown SGLT2 inhibitors can decrease HbA1c, proteinuria, brain natriuretic peptide (BNP), and blood pressure, and modestly improve ejection fraction in heart failure. Adverse effects include urinary tract infection and fungal genital infections (due to glucosuria), headache, diarrhea, vomiting, and euglycemic diabetic ketoacidosis (eDKA).
The mechanism of SGLT2-associated eDKA is not completely understood. However, diagnostic criteria of eDKA are similar to classic DKA. The most notable difference is the absence of hyperglycemia in eDKA that is typically seen in classic DKA. DKA occurs during a relative or absolute state of insulin deficiency with increased counter-regulatory hormones, including glucagon, corticosteroids and catecholamines. The imbalance of hormones leads to ketone formation and anion-gap metabolic acidosis. In the presence of SGLT2 inhibitors, glucosuria and diuretic effect lowers the serum glucose and decreases the overall insulin requirement. During periods of stress, such as illness, nausea, vomiting, fasting and surgery, the low insulin/high glucagon environment can lead to eDKA (see Figure 1 below).
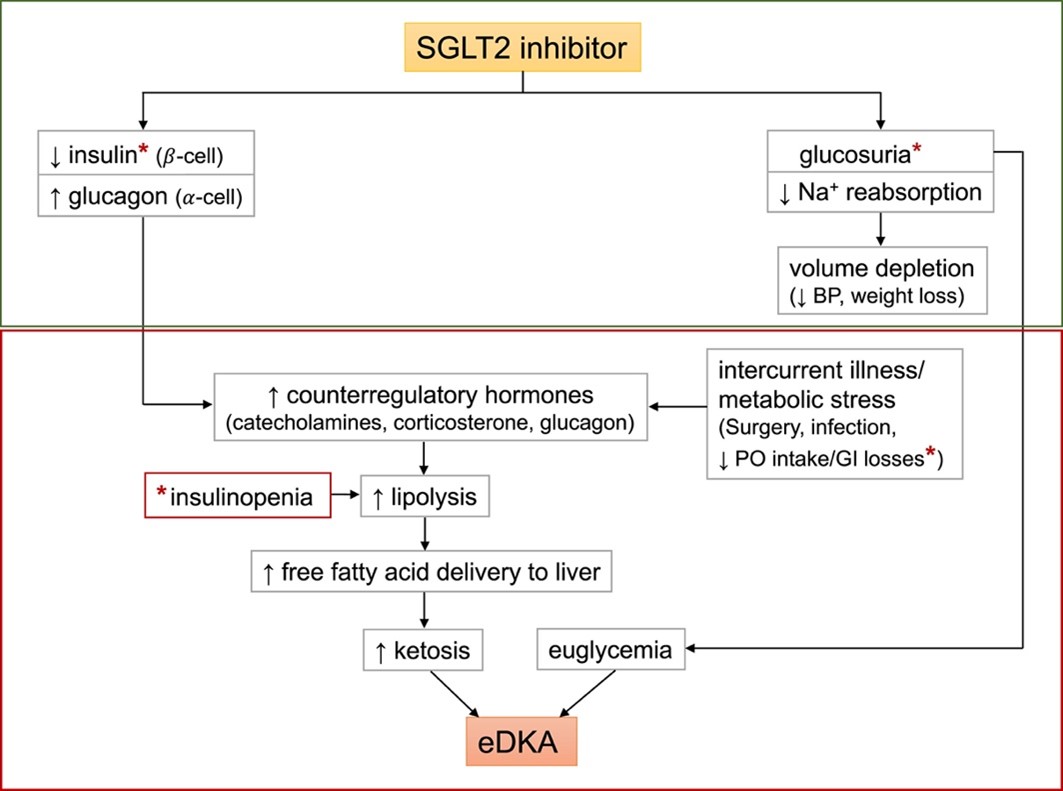
Figure 1. Proposed role of sodium-glucose cotransporter 2 (SGLT2) inhibition in euglycemic diabetic ketoacidosis (eDKA). Classic DKA results from insulin deficiency (absolute or relative) and concurrent increase in counter-regulatory hormones leading to ketosis, hyperglycemia, and osmotic diuresis. In contrast, SGLT2 inhibitor therapy in a well-compensated individual at baseline causes glucosuria, mild volume depletion, and lower serum glucose levels, associated with decreased insulin secretion. During times of intercurrent illness and/or metabolic stress, such as surgery or gastrointestinal illness, decreased carbohydrate intake coupled with lower serum glucose levels can further depress insulin secretion. This can ultimately lead to eDKA (red box). ∗Possible pathways of carbohydrate deficiency and causes of insulinopenia. Abbreviations: BP, blood pressure; PO, oral. (From: Wang KM and Isom RT. SGLT2 inhibitor-induced euglycemic diabetic ketoacidosis: A case report. Kidney Med. 2020;2:218-221. Used under Creative Commons License.)
A diagnosis of eDKA requires the following:
1. Blood glucose < 200 mg/dL
2. Ketonemia (serum beta-hydroxybutyrate greater than 3 mmol/L)
3. Anion gap metabolic acidosis:
a. Arterial pH < 7.3
b. Serum bicarbonate < 18 mEq/L
c. Anion gap > 10 mmol/L
Treatment of eDKA includes initiation of IV insulin infusion and dextrose solution, as well as IV fluid repletion and electrolyte management. The goal of therapy in eDKA is not to lower the serum glucose, but to replenish the insulin stores necessary to reduce ketone production. Monitoring of urine glucose can help determine residual SGLT2 inhibitor activity, and urine/serum ketones can guide efficacy of treatment.
Due to the frequency of reported perioperative eDKA events, the FDA updated its safety labeling in 2020 to recommend that empagliflozin, dapagliflozin, and canagliflozin be held for THREE days and ertugliflozin for FOUR days prior to elective procedures. In addition, a recent study from Massachusetts General Hospital has shown that anion gap metabolic acidosis develops in all patients when SGLT2 inhibitors were not held appropriately.
When patients on SGLT2 inhibitors present for urgent/emergent procedures, they must be monitored closely for the development of eDKA. Signs and symptoms of eDKA are non-specific and include nausea, vomiting, abdominal pain, fatigue, tachycardia, and tachypnea. Unfortunately, these are also signs of orthotopic heart transplant rejection and heart failure.
Due to increased evidence in the literature, candidacy and indications for SGLT2 inhibitor use are being expanded to include children ages ten years and older, non-diabetic patients with heart failure and congenital heart disease patients. This trend will increase the likelihood of pediatric cardiac anesthesiologists and pediatric anesthesiologists managing patients treated with SGLT2 inhibitors in the perioperative setting.
The correct answer is B. EDKA is characterized by the finding of increased serum and urine ketones, such as serum beta-hydroxybutyrate 5, in the presence of an increased anion-gap metabolic acidosis and relatively normal serum glucose levels.
REFERENCES
Grube PM, Beckett RD. Clinical studies of dapagliflozin in pediatric patients: a rapid review. Ann Pediatr Endocrinol Metab. 2022;27:265-72.
Steinhorn B, Wiener-Kronish J. Dose-dependent relationship between SGLT2 inhibitor hold time and risk from postoperative anion gap acidosis: a single-centre retrospective analysis. Br J Anaesth. 2023 Oct;131(4):682-6.
Chow E, Clement S, Garg R. Euglycemic diabetic ketoacidosis in the era of SGLT-2 inhibitors. BMJ Open Diab Res Care. 2023;11:e003666.
FDA revises labels of SGLT2 inhibitors for diabetes to include warnings about too much acid in the blood and serious urinary tract infections (fda.gov). 2022. Available at: https://www.fda.gov/drugs/drug-safety-and-availability/fda-revises-labels-sglt2-inhibitors-diabetes-include-warnings-about-too-much-acid-blood-and-serious. Accessed 7/17/2023.
