Author: Melissa Colizza, MD - Stollery Children’s Hospital -Edmonton AB Canada
A 1-week-old girl with severe pulmonary stenosis, intact ventricular septum, and moderate tricuspid valve regurgitation is status post balloon pulmonary valvuloplasty. Two hours later, the heart rate was 188, with an arterial blood pressure of 46/19 and oxygen saturation of 88%. A transthoracic echocardiogram demonstrates a large patent ductus arteriosus with left-to-right shunting, along with severe pulmonary and tricuspid regurgitation. Which of the following management options is MOST likely to improve the patient’s hemodynamics?
EXPLANATION
The most likely explanation for this patient’s hemodynamic instability is the development of a circular shunt. This is an uncommon physiological phenomenon in which blood from the systemic circulation enters the pulmonary circulation through the patent ductus arteriosus (PDA). The blood then flows into the right ventricle (RV) and the right atrium (RA) in a retrograde fashion through regurgitant pulmonary and tricuspid valves. From the RA, blood flows across an atrial septal defect (ASD) or a patent foramen ovale (PFO). Thus, a circular shunt occurs in the presence of a PDA, a right to left atrial shunt, and significant pulmonary and tricuspid valve insufficiency, following the pathway of least resistance. This is illustrated in Figure 1 below. Circular shunts usually lead to significant hemodynamic instability as both the pulmonary and tricuspid regurgitation effectively steal blood from both the systemic and pulmonary circulations, leading to low cardiac output and desaturation.
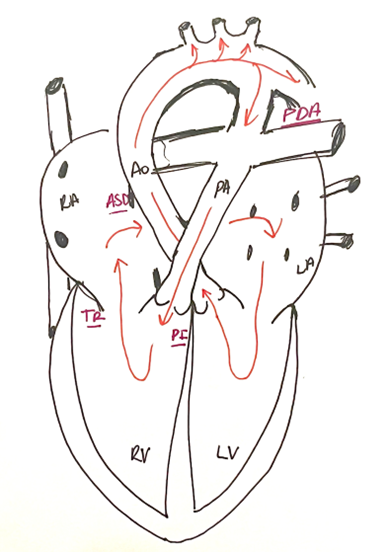 Figure 1. Pathway of blood flow in a circular shunt.
Figure 1. Pathway of blood flow in a circular shunt.
Circular shunts are typically described in the setting of severe Ebstein’s anomaly (EA), though they can also be seen after relief of right ventricular outflow tract obstruction (RVOTO) in patients with right-sided obstructive lesions. Bautista-Rodriguez et al. described two patients with severe pulmonary stenosis who developed circular shunt physiology following balloon dilation of the pulmonary valve. In both cases, the PDA failed to close, resulting in a low cardiac output state refractory to medical treatment after the procedure. PDA ligation was attempted to interrupt the circular shunt. However, upon complete occlusion of the PDA, the oxygen saturation decreased to 50%. At this point, the PDA was partially banded, targeting a systemic saturation of 70% and a mean systemic arterial pressure increase of greater than 20 mmHg. Both patients had good outcomes. The authors concluded that pulmonary insufficiency caused by balloon pulmonary valvuloplasty in the setting of tricuspid regurgitation and a small dysfunctional RV likely worsened RV function and favored retrograde flow in the presence of a PDA. Patients with severe EA who have a small, dysfunctional RV, significant TR, and a PDA may present similarly before intervention. Other susceptible patients include those with severe pulmonary stenosis (PS) and pulmonary atresia with intact ventricular septum (PA/IVS).
Medical management of a hemodynamically unstable circular shunt remains difficult and consists mainly of supportive treatment until an interventional or surgical procedure can take place. The goal is to optimize forward flow into the systemic and pulmonary circulation, mainly with inotropic support, reduction of systemic vascular resistance, and discontinuation of prostaglandin E1. Increasing pulmonary vascular resistance has also been described to decrease “steal” from the systemic circulation, although this might increase RV afterload and worsen pulmonary insufficiency. Definitive management depends on the underlying pathology. Options include PDA banding or ligation. In the case of EA, some options include pulmonary artery ligation or the Starne’s procedure.
The correct answer is A, patent ductus arteriosus ligation. This functions to eliminate retrograde flow into the pulmonary artery to the RV. Supportive measures with vasoactive agents or pulmonary vasodilators, such as milrinone and nitric oxide, are likely to be of short-term benefit without addressing the underlying mechanism of the circular shunt.
REFERENCES
Konstantinov IE, Chai P, Bacha E, et al. The American Association for Thoracic Surgery (AATS) 2024 expert consensus document: Management of neonates and infants with Ebstein anomaly. J Thorac Cardiovasc Surg. 2024;168(2):311-324. doi:10.1016/j.jtcvs.2024.04.018
Bautista-Rodriguez C, Rodriguez-Fanjul J, Moreno Hernando J, Mayol J, Caffarena-Calvar JM. Patent Ductus Arteriosus Banding for Circular Shunting After Pulmonary Valvuloplasty. World J Pediatr Congenit Heart Surg. 2017;8(5):643-645. doi:10.1177/2150135116655122
Andropoulos DB. Anesthesia for Congenital Heart Disease. 2nd ed. Wiley-Blackwell; 2010. Chapter 28: Anesthesia for Right-Sided Obstructive Lesions. Accessed August 12, 2024.
