Authors: Meera Gangadharan, MBBS, FAAP, FASA - Children’s Memorial Hermann Hospital, University of Texas Health Science Center, Houston, TX
AND
Destiny F. Chau, MD - Arkansas Children’s Hospital, UAMS, Little Rock, AR
An 18-year-old girl with a history of tricuspid atresia palliated with a Fontan presents for emergent craniotomy after a motor vehicle collision. The patient is treated with rivaroxaban for thromboprophylaxis. Which of the following medications is MOST appropriate to reverse the anticoagulant effects of rivaroxaban?
EXPLANATION
Patients with the Fontan physiology are at risk for thromboembolism secondary to “passive” pulmonary blood flow, venous hypertension, and hemostatic abnormalities. The risk for thromboembolism appears to be highest in the first three to twelve months following the Fontan operation, although a lower risk persists for life. The optimal anticoagulation strategy in children is still under debate. It is quite challenging with traditional medications such as warfarin and low-molecular-weight heparins, which require frequent blood draws for monitoring therapeutic levels and/or are administered subcutaneously. These patients are usually maintained on acetylsalicylic acid (ASA) despite the paucity of data on optimal dosing and aspirin resistance. A new class of anticoagulant drugs known as direct oral anticoagulants (DOACs) are now available, with significant advantages over current medications. These include dabigatran, rivaroxaban, apixaban, edoxaban and betrixaban. Only rivaroxaban and dabigatran are FDA-approved for use in children.
Rivaroxaban is an orally administered anticoagulant that binds directly and reversibly to free factor Xa (activated factor X) and prothrombinase-bound factor Xa (see Fig. 1). Rivaroxaban was approved by the Federal Drug Administration (FDA) in 2021 for two pediatric indications, including treatment of venous thromboembolism and risk reduction of recurrent venous thromboembolism and for thromboprophylaxis in pediatric patients with the Fontan palliation. Advantages of rivaroxaban include oral administration, no requirement for therapeutic monitoring, eliminating the need for repeated venipunctures, and fewer drug-drug and drug-food interactions. Rivaroxaban is excreted and eliminated by the kidneys and in the feces. P-glycoprotein and cytochrome P450 3A4 enzyme inducers and inhibitors will affect the pharmacology of rivaroxaban. Although the prothrombin time (PT) and the activated partial thromboplastin time (aPTT) are prolonged, there is no requirement to monitor these levels during therapy.
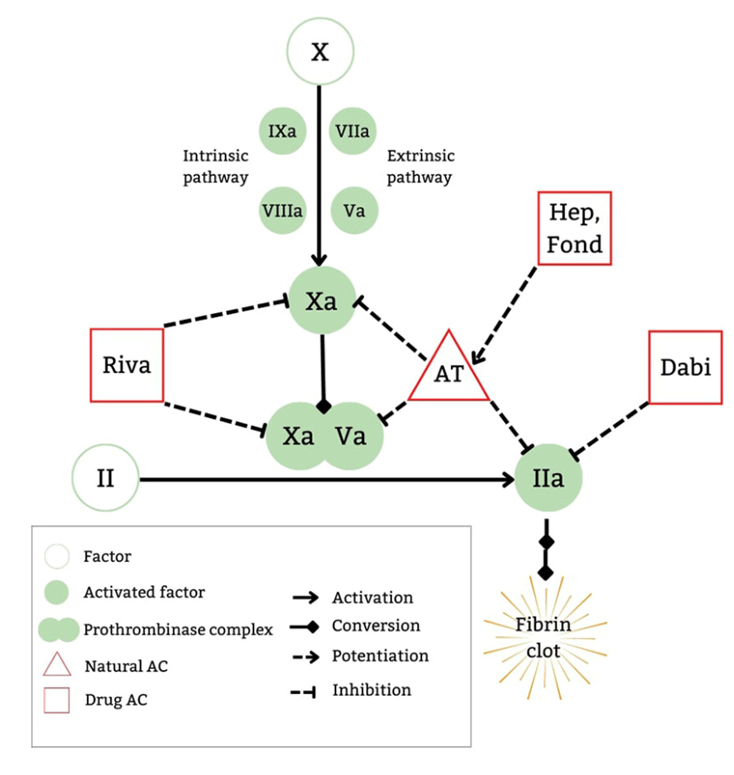 Fig. 1. Mechanisms of action of DOACs. From: Al-Ghafry M, Sharathkumar A. Direct oral anticoagulants in pediatric venous thromboembolism: Review of approved products rivaroxaban and dabigatran. Front Pediatr. 2022;10:1005098. Used under Creative Commons License.
Fig. 1. Mechanisms of action of DOACs. From: Al-Ghafry M, Sharathkumar A. Direct oral anticoagulants in pediatric venous thromboembolism: Review of approved products rivaroxaban and dabigatran. Front Pediatr. 2022;10:1005098. Used under Creative Commons License.
Rivaroxaban was compared to ASA in the randomized, multi-center, 2-part, open-label UNIVERSE study to evaluate its dosing regimen, safety, and efficacy. Part A was a dose-finding and safety determination, while Part B enrolled 100 subjects with the Fontan palliation. A total of 66 patients received rivaroxaban, and 34 received ASA for thromboprophylaxis. One patient in the rivaroxaban group experienced a major bleeding episode consisting of epistaxis. No major bleeding was reported in the ASA group. Six percent of the rivaroxaban group and nine percent of the ASA group experienced a clinically relevant non-major bleeding event. With regards to efficacy, one patient (2%) in the rivaroxaban group experienced a pulmonary embolism. In contrast, three participants (9%) in the ASA group reported thrombotic events, two venous thromboses and one ischemic stroke. Notably, the study was not powered to determine efficacy. The authors concluded that rivaroxaban had similar safety as ASA for thromboprophylaxis in patients with Fontan physiology.
Patients on rivaroxaban should have the medication stopped 24 to 48 hours before surgery, depending on the surgical procedure and renal function. If emergent reversal of anticoagulation by rivaroxaban is required, prothrombin complex concentrates (PCCs) or andexanet alfa may be administered. Andexanet alfa, an inactive recombinant factor Xa, has been approved for emergent reversal of rivaroxaban in adults. In a survey study, 44% of pediatric hematologists expressed that they prefer to administer andexanet alfa for emergent reversal of rivaroxaban in children versus PCCs.
Dabigatran is an orally administered direct thrombin inhibitor that has been FDA-approved for children older than three months of age. Dabigatran can be reversed with idarucizumab, an anti‐dabigatran monoclonal antibody fragment, which is approved for adults with major hemorrhage, and those requiring emergency surgery. Vitamin K antagonists (VKA) include warfarin, which works by inhibiting the vitamin K‐dependent coagulation factors, Prothrombin, Factor VII, Factor IX, and Factor X. Vitamin K should be given for reversal of VKA. However, it takes six hours for therapeutic effect after oral or intravenous administration of vitamin K. In emergent situations, four‐factor PCCs are recommended for rapid reversal of VKA if clinically indicated.
The correct answer is A. Andexanet alfa is recommended for reversal of rivaroxaban. Idarucizumab is recommended for reversal of dabigatran. Vitamin K is recommended for reversal of VKA,while four-factor PCCs are useful for emergent reversal.
REFERENCES
US Food and Drug Administration. FDA approves drug to treat, help prevent types of blood clots in certain pediatric populations. Accessed on January 4 2025 at
https://www.fda.gov/drugs/news-events-human-drugs/fda-approves-drug-treat-help-prevent-types-blood-clots-certain-pediatric-populations#:~:text=FDA%20has%20approved%20Xarelto%20(rivaroxaban,populations%20and%20for%20other%20uses
McCrindle BW, Michelson AD, Van Bergen AH, et al. Thromboprophylaxis for children post-Fontan procedure: Insights from the UNIVERSE Study J Am Heart Assoc.2021;10(22):e021765. doi:10.1161/JAHA.120.021765.
Al-Ghafry M, Sharathkumar A. Direct oral anticoagulants in pediatric venous thromboembolism: Review of approved products rivaroxaban and dabigatran. Front Pediatr. 2022;10:1005098. doi:10.3389/fped.2022.1005098
Doyle AJ, Crowley MP, Hunt BJ. Perioperative management of antithrombotic treatment in children. Paediatr Anaesth. 2019;29(5):405-413. doi:10.1111/pan.13511
