Author: Melissa Colizza, MD - Stollery Children’s Hospital - Edmonton, CA
A healthy ten-month-old, nine kg boy has just been weaned from cardiopulmonary bypass after repair of a ventricular septal defect. The results of a ROTEM performed after protamine administration are illustrated below. Which of the following treatments is MOST appropriate for hemostasis management in this patient?
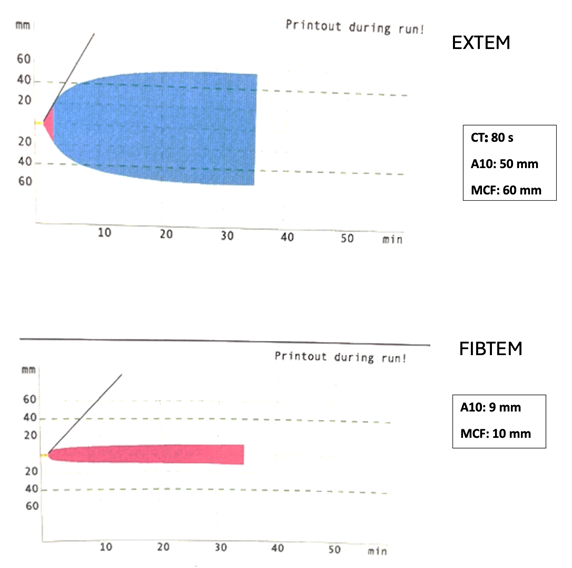
EXPLANATION
The neonatal hematologic system takes approximately one year to reach maturity. At birth, neonates have decreased platelet function, elevated von Willebrand factor concentration, lower levels of Factors II, VII, XI, X, XI, XII, pre-kallikrein, high-molecular-weight kallikrein, antithrombin, protein C, protein S, and decreased fibrinolytic activity. Overall, this results in a relative balance between pro-coagulants and anticoagulants. Therefore, neonates are at a high risk for both bleeding and thrombosis with any disruption in this delicate balance. Importantly, this risk is exacerbated in the setting of congenital heart disease and cardiac surgery with cardiopulmonary bypass (CPB).
CPB exerts multiple adverse effects on hemostasis, increasing the risk of bleeding. Hemodilution causes a relative deficit of all coagulation factor proteins, particularly fibrinogen. Blood exposure to the CPB circuit induces an inflammatory reaction, triggering the coagulation cascade via contact activation that results in thrombin generation, hyperfibrinolysis, and platelet activation, necessitating anticoagulation during CPB. Additional factors that increase the risk of bleeding during pediatric cardiac surgery with CPB include the inherent need for anticoagulation with unfractionated heparin, the use of moderate to deep hypothermia, and the length and complexity of cardiac surgical procedures. As a result, neonates and infants undergoing cardiac surgery with CPB are highly likely to require large volumes of blood products to correct coagulopathy.
Despite modern technological and pharmacological advances, management of hemostasis during pediatric cardiac surgery remains challenging. Viscoelastic testing (VET), including rotational thromboelastometry (ROTEM®), provides a comprehensive assessment of hemostasis at the point of care. ROTEM provides information on clot development from secondary hemostasis to clot lysis, including clot formation, clot firmness, and clot fibrinolysis. Basic ROTEM parameters include the following: 1) clotting time (CT), which is the time in seconds from the start of the test until significant levels (amplitude of 2 mm of clotting signal) of clot are detected; 2) the clot formation time (CFT), which is the time in seconds from the measurement of the CT until a fixed level of clot firmness (time between 2 mm and 20 mm amplitude of clotting signal); 3) the A10, which is the amplitude in mm of clotting signal 10 minutes after the CT; and 4) the maximal clot firmness (MCF), which describes the clot firmness and overall stability. Different assays are used in the ROTEM measurements, which target specific coagulation cascade components. The assays often used clinically include the INTEM, EXTEM, FIBTEM, and HEPTEM, which measure the intrinsic pathway, the extrinsic pathway, and the effect of fibrinogen and heparin, respectively.
These assays, as part of a transfusion algorithm, have been shown to decrease blood product transfusion and the incidence of major bleeding in adults. Data in the pediatric population is sparser and more equivocal. Faraoni et al. developed an algorithm to treat post-bypass coagulopathy in children using ROTEM. The authors found that the A10 on FIBTEM, as well as the CT and the A10 on EXTEM, were relevant parameters to guide hemostatic management. A prospective study by Naguib et al. studied the impact of using an institutional ROTEM-based transfusion algorithm on 28 infants and neonates. They found that patients in the ROTEM group received fewer overall platelet and cryoprecipitate transfusions than the control group and had higher hematocrit levels for the same amount of red blood cells transfused. In general, there is variability in the thresholds for transfusion of blood products and concentrates between institutions. Some of the published algorithms use the following: 1) a low A10 on FIBTEM to transfuse fibrinogen concentrate or cryoprecipitate; 2) a low A10 on EXTEM (with normal A10 on FIBTEM) to transfuse platelets; and 3) a prolonged CT on EXTEM to substitute clotting factors (i.e. factor concentrates or fresh frozen plasma).
The correct answer is A. The ROTEM illustrated above demonstrates a normal A10 and MCF on FIBTEM and a normal CT, A10, and MCF on EXTEM, suggesting normal hemostasis. Thus, this patient would not necessarily require any blood products, especially with no evidence of bleeding. However, it’s important to remember that ROTEM is a tool to assess whole blood coagulation, and the decision to transfuse blood products or factor concentrates hinges on other factors including clinical context, surgical variables, and institutional practices.
REFERENCES
Hartmann J, Hermelin D, Levy JH. Viscoelastic testing: An illustrated review of technology and clinical applications. ResPractThrombHaemost. 2023;7:e100031. doi.org/10.1016/j.rpth.2022.100031
Downey L and Faraoni D. Coagulation, Cardiopulmonary Bypass and Bleeding. In: Andropoulos DB, Mossad EB, Gottlieb EA, eds. Anesthesia for Congenital Heart Disease. Fourth edition. John Wiley & Sons, Inc.; 2023: 377-400.
Naguib AN, Carrillo SA, Corridore M, et al. A ROTEM-guided algorithm aimed to reduce blood product utilization during neonatal and infant cardiac surgery. J Extra Corpor Technol. 2023;55(2):60-69. doi:10.1051/ject/2023017
Faraoni D, Willems A, Romlin BS, Belisle S, Van der Linden P. Development of a specific algorithm to guide haemostatic therapy in children undergoing cardiac surgery: a single-centre retrospective study. Eur J Anaesthesiol. 2015;32(5):320-329. doi:10.1097/EJA.0000000000000179
