Author: Komal Kamra, MBBS, MMI
Clinical Professor
Department of Anesthesiology, Perioperative and Pain Medicine Stanford University Medical Center
The American Heart Association (AHA) defines cardiomyopathies as “ heterogeneous group of diseases of the myocardium associated with mechanical and/or electrical dysfunction that usually (but not invariably) exhibit inappropriate ventricular hypertrophy or dilatation and are due to a variety of causes that frequently are genetic.”(1) Based on the phenotype, cardiomyopathies can be further divided into four major categories as hypertrophic, dilated, restrictive, and non-compacted cardiomyopathies, among others. These cardiomyopathies may be liable for heart failure and eventually heart transplantation in the pediatric population. The anesthesiologist may encounter cardiomyopathy patients presenting for diagnostic evaluations or surgical interventions. Diagnostic evaluations include echocardiography, cardiac MRI, CT angiography, nuclear imaging, and myocardial or skeletal muscle biopsies. If medical treatment fails, the cardiomyopathy patient may subsequently require surgical intervention for the underlying disease as indicated by the pathology, mechanical circulatory support, and heart transplantation. Other interventions may encompass biventricular pacemaker insertion for cardiac resynchronization therapy and insertion of an automatic implantable cardioverter defibrillator to minimize the risk of sudden death due to ventricular arrhythmias. This review outlines the basic echocardiographic features useful in diagnosing, assessing severity, and channeling clinical management of the four major cardiomyopathies mentioned above.
HYPERTROPHIC CARDIOMYOPATHY
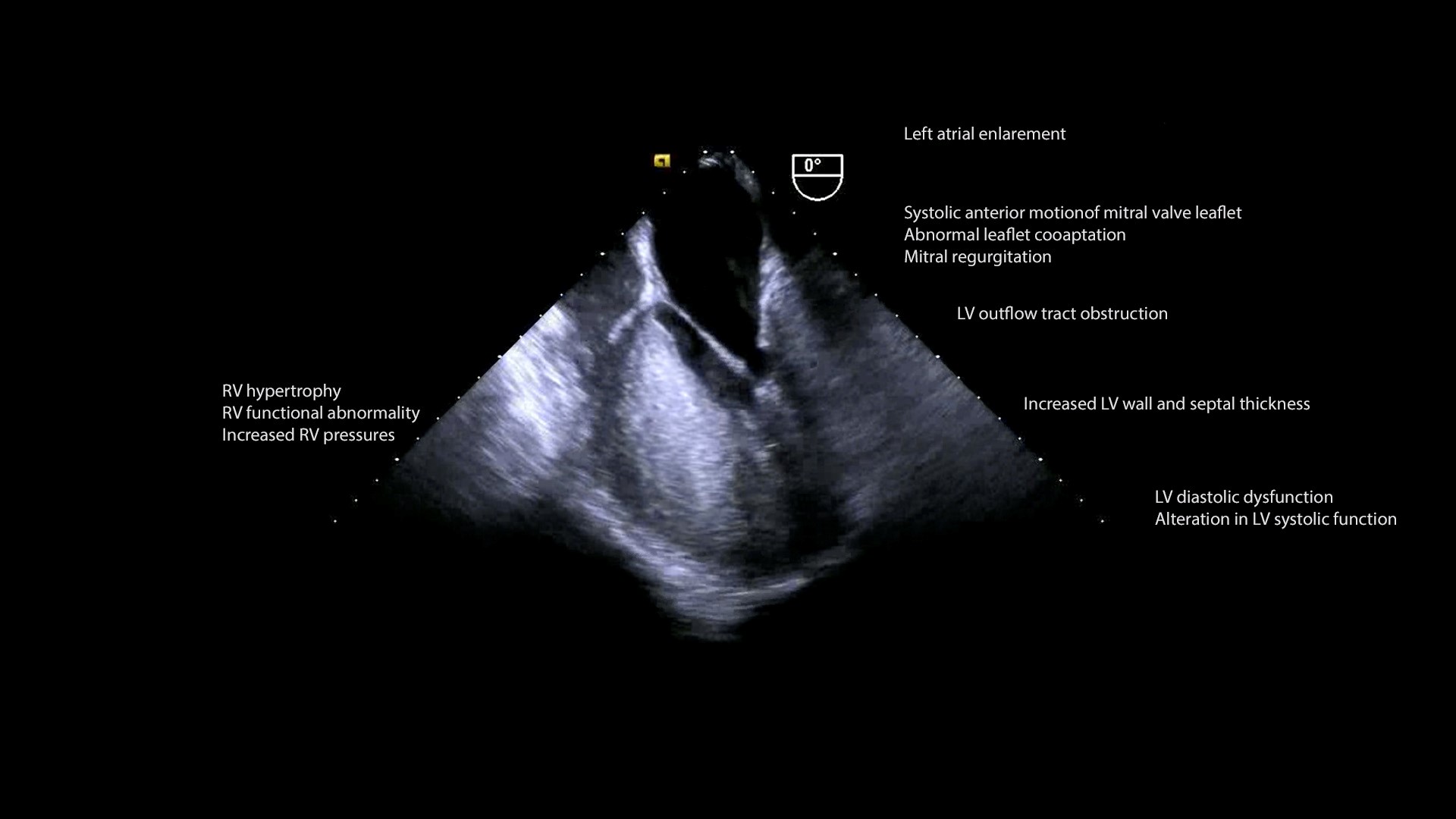
Hypertrophic cardiomyopathy (HCM) is an autosomal dominant genetic disorder with an incidence of about 0.5/100,000 in the pediatric population. It is defined as increased left ventricular (LV) wall thickness in the absence of dilation that cannot be specifically attributed to structural or loading abnormalities of the heart, notably valvular lesions, hypertension, and congenital heart disease. Per AHA guidelines, in pediatric patients, “increased LV wall thickness is defined as wall thickness > 2 standard deviations above the mean (z score > 2) for age, sex, or body size.” The pathophysiology of HCM (Figure 1, Video 1) comprises left ventricular outflow tract obstruction (LVOTO), mitral regurgitation (MR), myocardial ischemia, diastolic and autonomic dysfunction.(2)
Figure 1. Pathophysiological consequences of Hypertrophic cardiomyopathy
HCM can be subdivided into familial and non-familial forms. The hypertrophy is frequently exhibited as asymmetrical hypertrophy of the interventricular septum and sometimes also affects the LV free wall. LV hypertrophy and wall thickness are evaluated by echocardiography. Linear dimensions, mass, and volume are utilized for assessment of LV size. These measurements are indexed to body surface area, and are made both at the end-systole (frame at which the mitral valve closes or at the end of T-wave), and end-diastole (frame right before the mitral valve opens or beginning of QRS complex).
The mitral apparatus is impacted in HCM; mitral valve leaflets can elongate, annular calcification can lead to a reduced systolic excursion, chordal rupture or excessive laxity may occur, and the papillary muscle may be anteriorly displaced, bifid, hypertrophied, or have anomalous insertion into the leaflets. Systolic anterior motion (SAM, Video 1B) of the mitral valve (MV) may manifest in HCM as the hyperdynamic LV generates a Venturi effect, which pulls the redundant and mobile anterior MV leaflet into the LVOT during systole. This shifts the coaptation point from the tips of the two leaflets to the body of the anterior leaflet and tip of the posterior leaflet. Failure of proper coaptation precipitates mid to late systolic posteriorly-directed MR jet. Other anomalies affecting the MV, such as prolapse, calcification, or fibrosis can contribute to MR, with a jet which spans the entire systole and is anteriorly directed.
Legend for Video 1B: Zoomed ME LAX view color compare. Left, 2D imaging showing systolic anterior motion (SAM) of the anterior mitral leaflet and asymmetric septal hypertrophy. Right, color flow Doppler imaging showing left ventricular outflow tract obstruction (LVOTO) and mitral regurgitation.
LVOTO results from hypertrophy of the septum, abnormal placement of papillary muscles, and MV leaflet anomalies. Obstruction may also be evident within the LV cavity or at the mid ventricular level. The LVOTO is dynamic and affected by loading conditions and contractility.
HCM is characterized with increased systolic ejection fraction (EF) and LV end-diastolic pressure but decreased diastolic dysfunction. Impaired relaxation, rather than decreased ventricular compliance, is a predominant catalyst for the diastolic dysfunction. Systolic dysfunction left atrial (LA) enlargement, and atrial fibrillation may occur as the disease progresses.
Left ventricular function
Qualitative evaluation of LV systolic function:
A qualitative estimate of LV systolic function can be done by visual estimation of ejection fraction, namely mild (EF 41-55%), moderate (EF 31-40%), and severe (EF < 30%) dysfunction. Normal EF is > 55%.
Quantitative evaluation of LV systolic function:
Main parameters used for quantitative evaluation of LV systolic function are as follows:
Fractional Shortening (FS):
- calculated using M-mode or 2D imaging
- FS (%) = (EDD – ESD)/EDD x 100 EDD, end-diastolic dimension; ESD, end-systolic dimension normal FS = 26-45%
- On the basis of the FS, the LV function is subdivided into mild dysfunction (FS 20-25%), moderate dysfunction (FS 15-19%), severe dysfunction (FS <14%), and hyperdynamic (FS >45%).
Ejection fraction (EF):
- calculated using Biplane Simpson, Area-length, and 3D modalities
- EF = (EDV-ESV)/EDV x 100 EDV, end-diastolic volume; ESV, end-systolic volume normal EF= 56-78%
Fractional area change (FAC):
- Area measurement utilized to assess global LV systolic function
- FAC (%) = (EDA-ESA)/EDA x 100 EDA, end-diastolic area; ESA, end-systolic area
Left atrial size
LA enlargement is precipitated by MR and elevated LV filling pressures. LA size can be assessed qualitatively by TEE but is best measured by TTE.
Linear measurement
LA anteroposterior dimension is the distance between the posterior aortic wall and the posterior LA wall measured at the level of the aortic sinuses. Both 2D and M-mode are utilized for this measurement.
LA area
LA area is measured in an apical 4-chamber view.
LA volume
Major and minor axis diameter of LA is measured in the apical 4-chamber and 2-chamber view at end-systole. LA volume is then calculated by the biplane area method and indexed to body surface area. Normal values for body surface area are published for pediatric patients. This can also be reliably quantitated by 3D echocardiography.
DILATED CARDIOMYOPATHY
Dilated cardiomyopathy (DCM) is characterized by the presence of ventricular dilation along with systolic dysfunction without the evidence of abnormal loading scenarios, such as valvar abnormality, congenital heart disease, hypertension, or coronary artery disease. LV wall thickness is usually normal. Dilated cardiomyopathy may also be associated with right ventricular dysfunction and dilation.(1)
Based on the etiology, DCM can be subdivided into familial/genetic and non-familial/ non-genetic types. Genetic type can be associated with mutations and inborn errors of metabolism. Non-genetic type can be a result of myocarditis, cardiotoxic drugs, immune mediation, endocrine and nutritional deficiencies, and tachycardia, among others.(3)
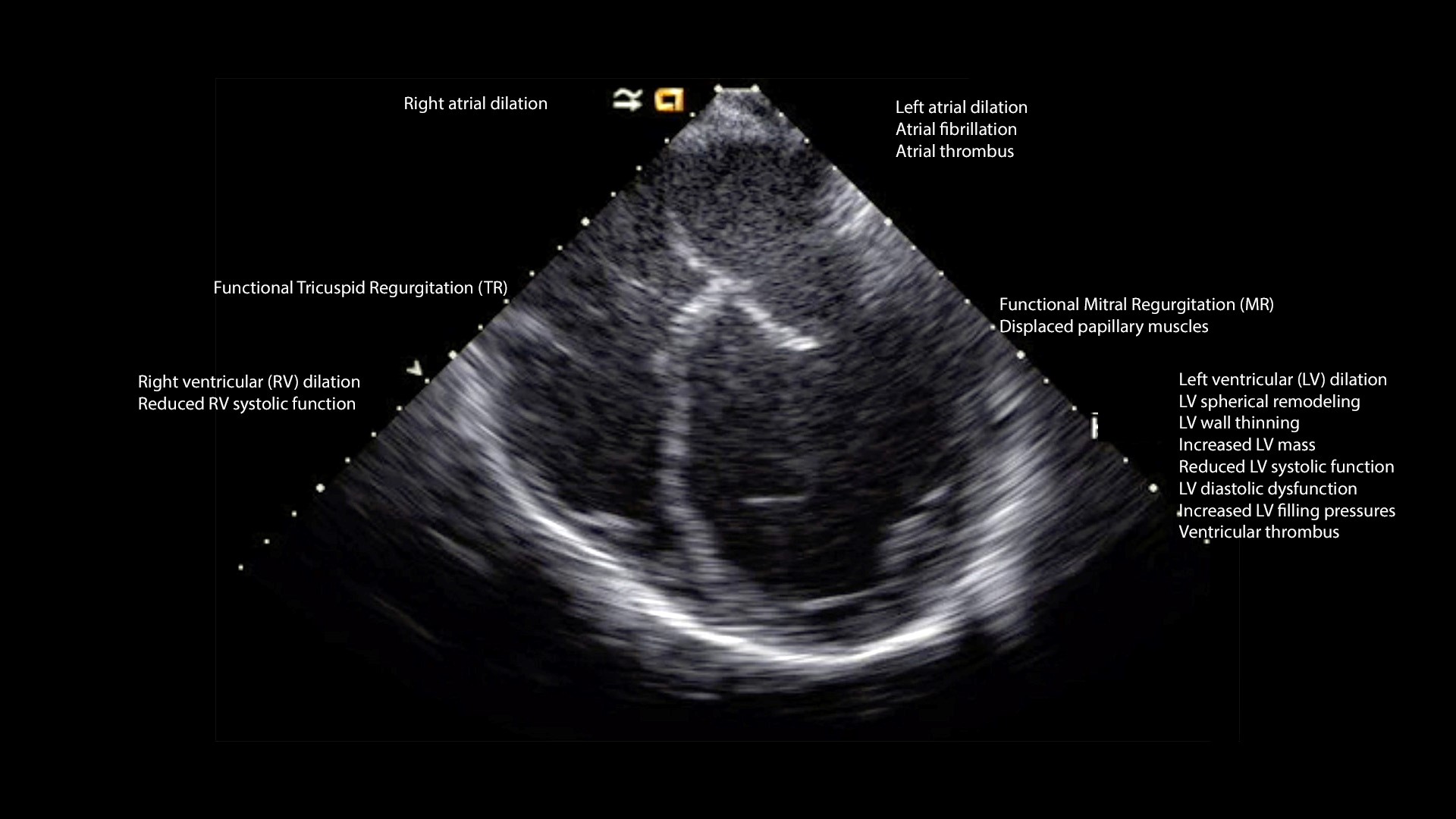
The predominant echocardiographic features in DCM are described below (Figure 2, Video 2).
Figure 2. Echocardiographic features of dilated cardiomyopathy
Dilated left ventricle and spherical remodeling
The assumes a spherical shape in DCM as compared to its normal ellipsoidal shape. The alteration in shape results in the sphericity index value of about one. Sphericity index is the ratio of the diastolic diameter of the LV measured in a short-axis view to the diastolic length of the LV measured in the apical four-chamber view. In normal pediatric patients, the value is less than one.
Diminished left ventricle systolic function
Ejection fraction and shortening fraction are reduced in patients with DCM.
LV wall thinning and LV mass
Even though DCM is accompanied by LV wall thinning, due to its increased size, LV mass is enhanced.
LV thrombus
The reduced ventricular systolic function in DCM can lead to stasis and thrombus formation. Stasis presents as a spontaneous echo contrast within the cavity and intracardiac thrombus as a filling defect.
LV diastolic dysfunction and elevated LV filling pressures
Echocardiographic parameters used to ascertain the LV diastolic function and filling pressures are mitral inflow, pulmonary vein, mitral annular systolic and diastolic tissue Doppler velocities, and mitral annular color M-mode propagation velocity.
LV dyssynchrony
LV systolic and diastolic mechanical dyssynchrony can be detected in pediatric patients with DCM. This can be evaluated at atrioventricular, interventricular, and intraventricular levels. Mitral inflow Doppler velocity assists in evaluating atrioventricular mechanical dyssynchrony. Interventricular mechanical dyssynchrony can be quantified from pulse Doppler interrogation across the outflows. M-mode echocardiography, tissue Doppler, speckle tracking, strain imaging, and 3D echocardiography can also be used to analyze the intraventricular mechanical dyssynchrony.
Left atrial dilation
Left atrial dilation can be attributed to diastolic dysfunction, functional MR, and atrial arrhythmias. As a corollary to stasis, left atrial thrombus formation can occur.
Right ventricle (RV)
RV as well as right atrial remodeling and dilation can occur due to primary myocardial disease or as a consequence of LV dysfunction and pulmonary hypertension. Reduction in the systolic and diastolic function of the RV may be due to interventricular interaction. Functional tricuspid regurgitation may accompany RV remodeling and dysfunction.
Mitral apparatus
Functional MR is a byproduct of LV dilation and remodeling. Remodeling involves displacement of papillary muscle, dilation of the MV annulus, and improper apposition the leaflets.
Pulmonary hypertension
Increased LV and LA filling pressures cause a passive increase in pulmonary venous pressure. This increment in pulmonary venous pressure overtime promotes pulmonary vasculature remodeling and secondary pulmonary hypertension.(4)
RESTRICTIVE CARDIOMYOPATHY
Primary restrictive cardiomyopathy (RCM) is rare cardiomyopathy contributing to nearly 4.5% cases of cardiomyopathy. Its main features are summarized by AHA “as a normal or decreased volume of both ventricles associated with biatrial enlargement, normal LV wall thickness and AV valves, impaired ventricular filling with restrictive physiology, and normal (or near-normal) systolic function.” RCM can either be idiopathic or a result of infiltrative, storage and systemic diseases.(1)
The main echocardiographic parameters used for assessment of RCM are Doppler velocities across mitral and tricuspid inflows, and lateral and medial mitral annular tissue Doppler velocities. Mitral inflow Doppler shows increased E-velocity and E/A ratio (> 2.5), and reduced deceleration time (< 150 ms). Respirophasic variation in mitral inflow velocity is absent. Tissue Doppler velocities (e’) are diminished. Analogous to normal patients, lateral tissue Doppler velocities are higher than medial tissue Doppler velocities. E/e’ ratio is enhanced (>14), while iso-volumetric relaxation time is diminished (<50 cm).
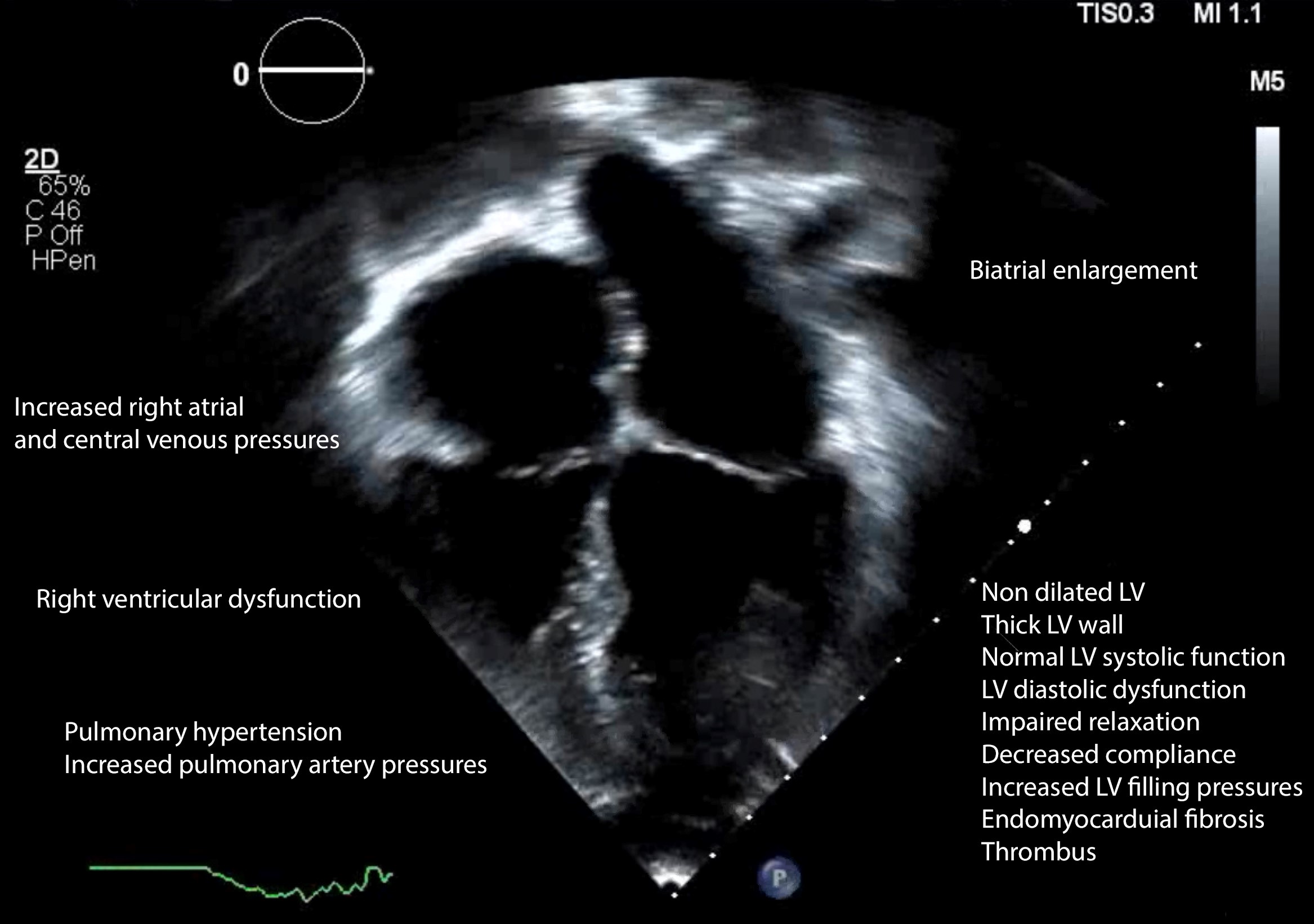
Additional information is obtained from a pulsed Doppler assessment of hepatic and pulmonary veins and superior and inferior vena cava (Figure 3, Video 3). Hepatic vein flow demonstrates large A-wave and inspiratory diastolic flow reversal. Pulmonary vein Doppler depicts enhanced A-wave as well.
LA volume index, septal shift with respiration, and pulmonary artery pressure are also evaluated. Biatrial enlargement is exhibited in RCM.
Other echocardiographic features in RCM include normal to increased LV wall thickness, normal to decreased ventricular volume, impeded/increased ventricular filling, diastolic dysfunction, impaired ventricular relaxation and compliance, and conserved systolic function.(5)
Figure 3. Echocardiographic features of restrictive cardiomyopathy
Non-compaction Cardiomyopathy
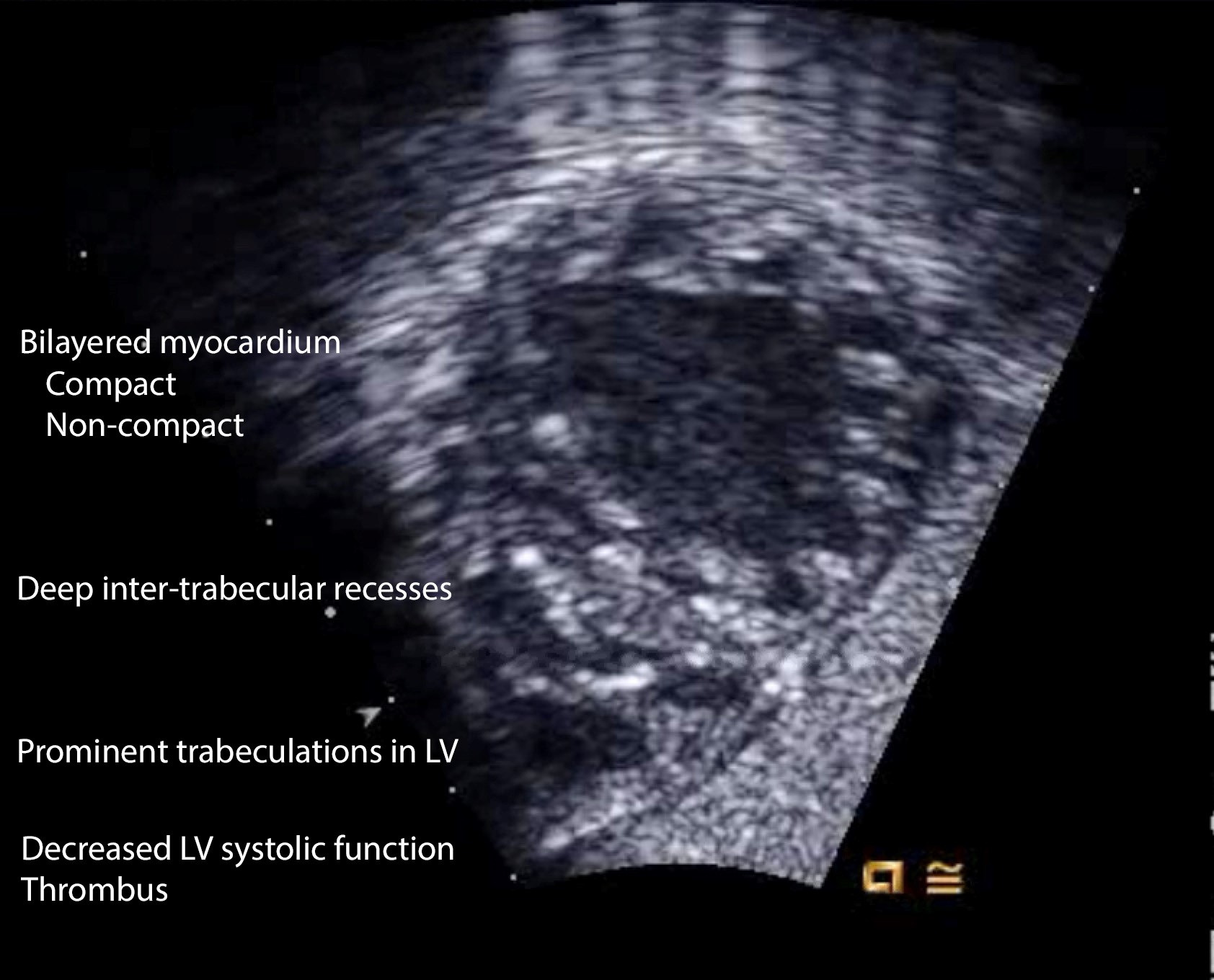
Salient features of non-compaction cardiomyopathy (NCCM) are > 3 enlarged ventricular trabeculations and deep inter-trabecular recesses in the left ventricle. These recesses derive the blood from the ventricular cavity and are not connected to the epicardial coronary system. The myocardium has two layers – an epicardial layer that is thin and compact, and endocardial layer that is thick and non-compacted (Figure 4, Video 4). The compaction of the myocardium is a natural process that occurs during 3-4 month of fetal life. Compaction is followed by the growth of spiral fibers, which account for the twisting motion of the ventricle. Myocardial dysfunction ensues because of the omission of compaction and resultant contraction abnormalities. Sub-endocardial ischemia is evident. The blood in the inter-trabecular recess accompanied by myocardial dysfunction results in thrombus formation and embolism. Prominent trabeculations and recesses are observed at the apex, mid-lateral, and mid-inferior walls of the heart. Contrast helps distinguish compact (apparent) from non-compact myocardium (concealed) and also facilitates thrombus recognition. Clinical ramifications of NCCM are arrhythmias, heart failure, and embolic stroke.
Figure 4. Echocardiographic features of non-compaction cardiomyopathy
Two main echocardiographic criteria (Chin’s and Jenni’s) are used to diagnose NCCM. The measurements for Chin’s criterion are made during end-diastole. For this criterion, the thickness of compact myocardium (X) and the thickness of compact and non-compact myocardium (Y) is measured. X/Y is <0.5 in non-compacted myocardium. The measurements are made in multiple transthoracic views. In Jenni’s criterion, measurements are made in SAX view during end-systole. The ratio of the thickness of non-compact to the thickness of the compact layer is >2 in non-compacted cardiomyopathy. Color Doppler assessment of inter-trabecular recesses will reveal their connection to the ventricular cavity.(6)
This review summarizes the main echocardiographic features of the four principal cardiomyopathies found in children and should serve as a guide to the anesthesiologists involved in clinical care of these patients.
References
- Maron BJ, Towbin JA, Thiene G, Antzelevitch C, Corrado D, Arnett D, et al. Contemporary definitions and classification of the cardiomyopathies: an American Heart Association Scientific Statement from the Council on Clinical Cardiology, Heart Failure and Transplantation Committee; Quality of Care and Outcomes Research and Functional Genomics and Translational Biology Interdisciplinary Working Groups; and Council on Epidemiology and Prevention. Circulation. 2006;113(14):1807-16.
- Gersh BJ, Maron BJ, Bonow RO, Dearani JA, Fifer MA, Link MS, et al. 2011 ACCF/AHA guideline for the diagnosis and treatment of hypertrophic cardiomyopathy: executive summary: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. Circulation. 2011;124(24):2761-96.
- Konta L, Franklin RC, Kaski JP. Nomenclature and systems of classification for cardiomyopathy in children. Cardiol Young. 2015;25 Suppl 2:31-42.
- Abate E. PB, Porto A. Basic Echocardiography in Dilated Cardiomyopathy. In: Pinamonti B. SG, editor. Clinical Echocardiography and Other Imaging Techniques in Cardiomyopathies. Cham: Springer; 2014.
- Welch TD, Ling LH, Espinosa RE, Anavekar NS, Wiste HJ, Lahr BD, et al. Echocardiographic diagnosis of constrictive pericarditis: Mayo Clinic criteria. Circ Cardiovasc Imaging. 2014;7(3):526-34.
- Song ZZ. Echocardiography in the diagnosis left ventricular noncompaction. Cardiovasc Ultrasound. 2008;6:64.
Suggested Reading list
- Lee TM, Hsu DT, Kantor P et al. Pediatric Cardiomyopathies. Circ Res. 2017; 121: 855-73.
- Jan MF, Tajik AJ. Modern Imaging Techniques in Cardiomyopathies. Circ Res. 2017; 121: 874-91.
- Lipshultz SE, Law YM, Asante-Korang A et al. Cardiomyopathy in Children: Classification and Diagnosis: A Scientific Statement From the American Heart Association. Circulation. 2019; 140: e9-e68
- Lipshultz SE, Cochran TR, Briston DA et al. Pediatric cardiomyopathies: causes, epidemiology, clinical course, preventive strategies and therapies. Future Cardiol. 2013; 9: 817-48.