Lisa A. Caplan, MD and Wanda C. Miller-Hance, MD
Texas Children’s Hospital and Baylor College of Medicine, Houston, Texas
(Please note the Tables at the end of this document)
INTRODUCTION
The coronary arteries are the vessels that provide blood supply to the myocardium, being crucial for the maintenance of cardiac output and thus bodily functions. Anomalies of the coronary arteries can be congenital or acquired (1). In some cases, the congenital coronary anomalies (CCAs) can be associated with other types of congenital heart disease (CHD). Acquired coronary anomalies may be a consequence of a genetic syndrome, disease state, or due to surgical interventions that involve these vessels. Williams syndrome, a deletion of chromosome 7q11.23 which causes an elastin arteriopathy, can potentially result in smooth muscle hyperplasia, luminal vessel narrowing, and ultimately coronary arterial stenosis (2).
Although in general CCAs represent a relatively small component of pediatric heart disease, it is important for the anesthesiologist to be familiar with these pathologies and important aspects of their assessment, including the contributions of echocardiography, and, of intraoperative imaging to their care. This review focuses on the role of transesophageal echocardiography (TEE) in the assessment of the coronary arteries and most common pathologies of these vessels in children. An overview of recommended TEE views will be described with emphasis on preoperative and post repair evaluation for each of these anomalies. The role of epicardial imaging (E-echo) in this setting is also briefly addressed.
NORMAL CORONARY ARTERY ANATOMY
The two main coronary arteries that supply the heart are the right coronary artery (RCA) and the left main coronary artery (LMCA) (Figure 1).
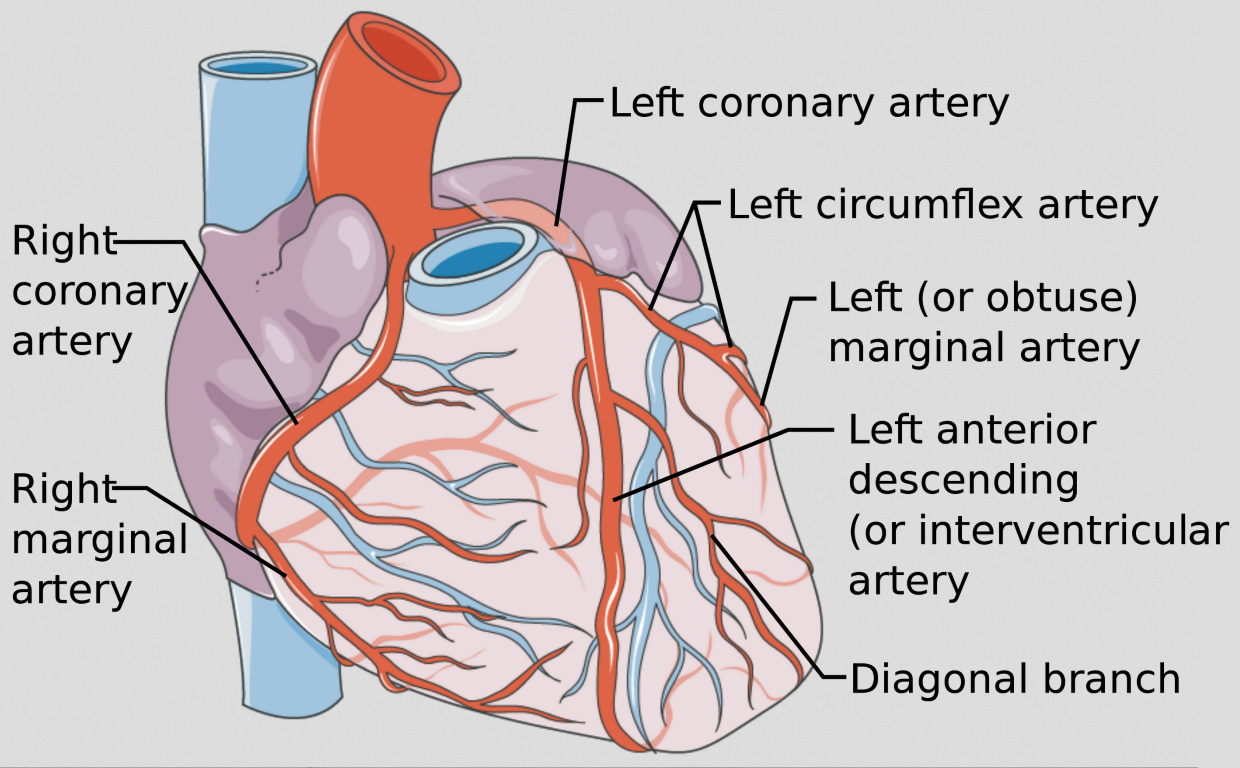
Figure 1 Coronary Artery Anatomy
The distribution of the coronary arteries and pattern of blood supply to the myocardium is shown. Source: Coronary vessels, with annotated arteries by Mikael Häggström, used under Creative Common License CC BY 3.0. No changes have been made to this image.
These vessels originate just above the aortic cusps from their respective sinuses of Valsalva adjacent to the subpulmonary infundibulum. In the normal heart the coronary arteries originate from the sinuses facing the main pulmonary artery (MPA). Although there is known significant variability in the normal coronary circulation, in general the distribution of the coronary arteries and pattern of blood supply to the myocardium can be considered as noted in Table I.
TRANSESOPHAGEAL ECHOCARDIOGRAPHIC EVALUATION OF THE CORONARY ARTERIES
As a background on the subject of TEE evaluation in children and all patients with CHD, the reader is referred to published guidelines that describe in detail the recommended 28 main tomographic views that are to be used in a comprehensive examination (Figure 2) (3). They serve as the basis for the discussion that follows.
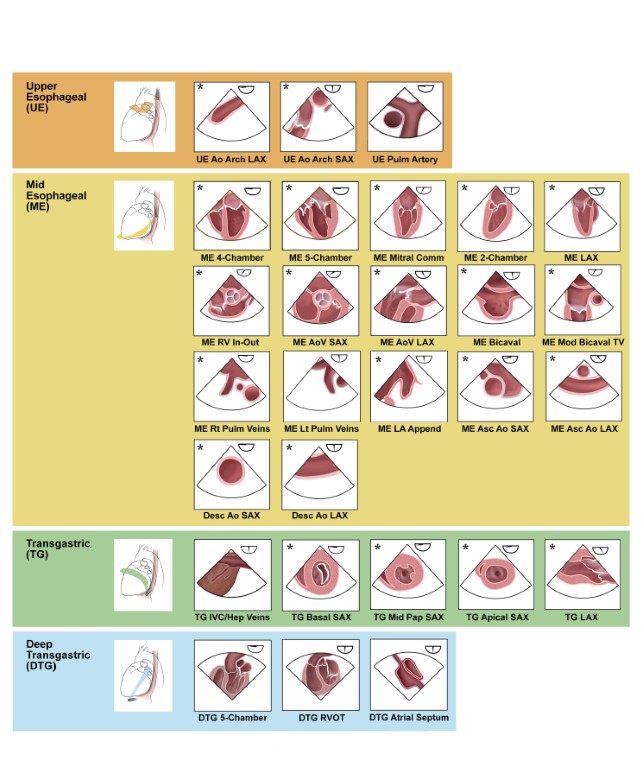
Figure 2 TEE Views for the Comprehensive Pediatric and Congenital Examination
Main tomographic views recommended in the guidelines for the comprehensive TEE examination in children and all patients with CHD (3). Panels marked with an asterisk correspond to the TEE views that are also part of the adult comprehensive TEE guidelines (22). Source: Texas Children’s Hospital, with permission.
For focused assessment of coronary artery origin and proximal course
In the evaluation of the main coronary arteries and related abnormalities, a focused TEE assessment is essential to address origin, course, and blood flow in these vessels, as well as global and segmental ventricular function. It is also important to evaluate for possible associated findings as surrogates of coronary insufficiency (e.g., mitral regurgitation).
A combination of multiple two-dimensional (2D) views and sweeps is required to image normal coronary arteries and particularly, in the pre and postoperative assessment of coronary artery anomalies. Modalities such as color Doppler and spectral should complement the examination (Figures 3 and 4; Videos 1 and 2).
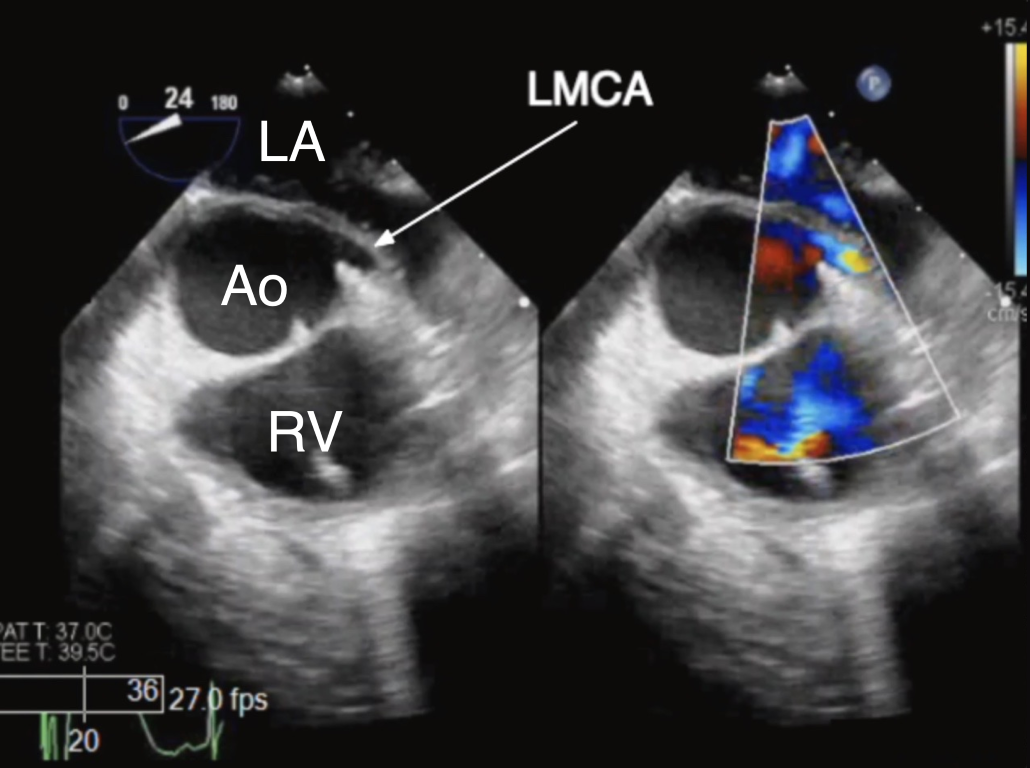
Figure 3 TEE Imaging of the Left Main Coronary Artery
ME AoV SAX 2D (left panel) and color Doppler (right panel) view of the left main coronary artery (LMCA) as it originates normally from the left coronary cusp. Ao aorta, LA left atrium, RV right ventricle
Video 1 TEE Imaging of Left Main Coronary Artery
ME AoV SAX view displaying the left main coronary artery (LMCA) as it originates normally from the left aortic sinus of Valsalva by 2D imaging and color flow Doppler
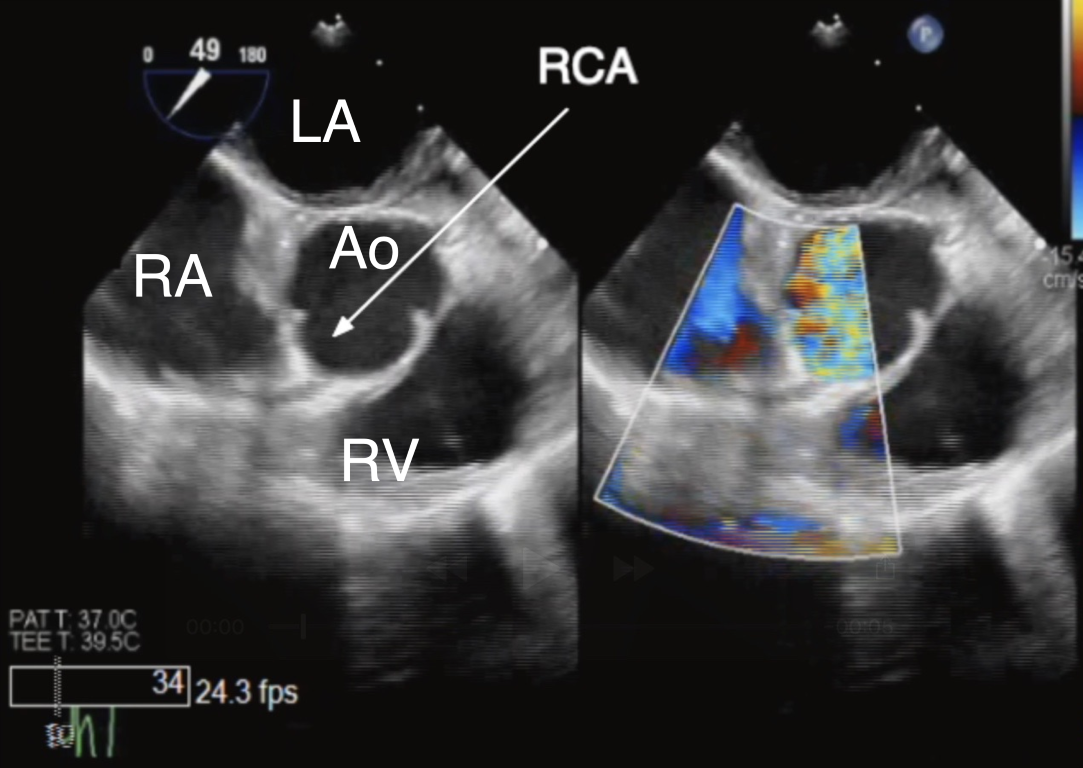
Figure 4 TEE Imaging of the Right Coronary Artery
ME AoV SAX 2D (left panel) and color Doppler (right panel) view of the right coronary artery (RCA) as it originates normally from the right coronary cusp. Ao aorta, LA left atrium, RA right atrium, RV right ventricle
Video 2 TEE Imaging of the Right Coronary Artery
ME AoV SAX view displaying the right coronary artery (RCA) as it originates normally from the right aortic sinus of Valsalva by 2D imaging and color flow Doppler
The TEE views considered most useful in the evaluation of origins and proximal segments of the coronary arteries are noted in Table II. It should be emphasized that modifications of these views and additional cross-sections may be necessary, and at times essential, in this assessment depending on the particular anatomy.
To perform a color Doppler examination of the coronary arteries, the image sector should be adjusted to the size of the vessel of interest allowing for an increased frame rate. The color Doppler scale is usually adjusted to the identify low-velocity flows (20-40 centimeter per second) (4). Normal color Doppler flows are laminar and antegrade in systole and diastole, aliasing or requiring a higher color scale suggests possible obstruction.
To perform a pulsed-wave Doppler examination the sample volume should be positioned over the proximal portion of the coronary artery to be interrogated (4). Normal spectral Doppler flow patterns for the left and right coronary arteries consists of a biphasic pattern with a late systolic peak followed by diastolic flow. The pattern should return to baseline between systole and diastole with no flow reversal. Care should be taken to ensure that the Doppler beam is parallel to the coronary artery flow as if otherwise, this may introduce errors in interpreting coronary artery velocities or flow patterns.
For functional evaluation of the left ventricle
The blood supply to the left ventricle (LV) can be compromised in the setting of coronary anomalies resulting in papillary muscle ischemia, mitral valve regurgitation, ventricular dysfunction and even failure, and myocardial infarction. A thorough evaluation of LV function (global and regional) and affected anatomic structures can be performed using complementary TEE views (Figure 5, Table III,).
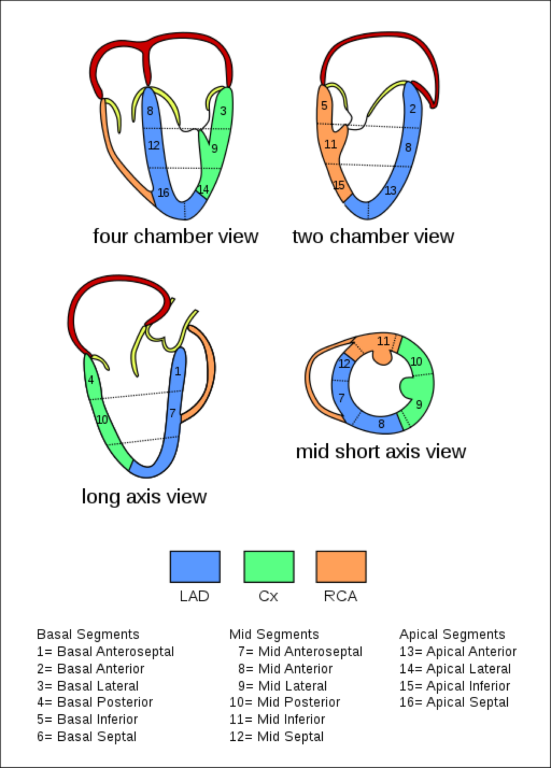
Figure 5 Myocardial Regions Perfused by the Major Coronary Arteries
The TEE views with corresponding myocardial regions supplied by the major coronary arteries are shown. Source: TEE Coronary Region (CardioNetworks ECHOPedia), used under Creative Common License CC BY-SA 3.0. No changes have been made to this image.
Obtaining systolic functional parameters such as shortening fraction, ejection fraction (EF), strain imaging, or volumetric assessment by three-dimensional echocardiography is time-consuming and not routinely applied in the intraoperative setting. Therefore, most of the functional LV evaluation relies on a visual qualitative estimation by assessing for changes in chamber volume and gross dimensions during the cardiac cycle, or systolic contractility (5). In the TG Mid Pap SAX view, all the main LV walls may be viewed for a gross qualitative assessment, in contrast to the 2 opposing walls in the ME 4-Ch view. Grossly normal LV function corresponds to an LVEF of 60%, mild hypokinesis to 50%, moderate hypokinesis to 40%, severe hypokinesis to 30%, and akinesis to 20%. A qualitative regional functional assessment requires identification of an abnormally contracting myocardial segment in the distribution of the coronary artery of interest. During systole the walls of the LV should normally move inwards and thicken. Abnormalities are categorized as: hypokinesia, when there is reduced LV wall segment systolic inward excursion or thickening; akinesia, when there is reduced or absent systolic excursion or absent thickening; and dyskinesia, when there is systolic outward excursion or lengthening or absent wall thickening. The number of abnormal segments suggests the extent of myocardial damage, and severity of the regional contractile abnormalities reflects the viability of the segment (6). The TG Mid Pap SAX allows for visualization of the myocardium perfused by all 3 coronary arteries. Abnormal findings should be confirmed in complementary views.
Congenital Coronary Artery Anomalies
Anomalous origin of a coronary artery from the opposite sinus of Valsalva
A congenital malformation of a coronary artery in most cases relates to its origin. In 0.21-5.79% of the population a coronary artery arises from a location outside the sinus of Valsalva (7, 8). A wide range of pathophysiologic consequences can result from these malformations ranging from no symptomatology to manifestations such as myocardial ischemia, arrhythmias, or sudden death (9). Some of these anomalies may not be identified until late adolescence or even adulthood.
Anomalous aortic origin of a coronary artery (AAOCA) represents roughly a 1/3 of all coronary artery anomalies and may involve any of the 3 coronary arteries (10). While many classification systems exist, the most serious subtypes that infer risk of sudden death or cardiac symptoms are: LMCA arising from the right sinus of Valsalva and RCA arising from the left sinus of Valsalva (Figure 6) (10, 11).
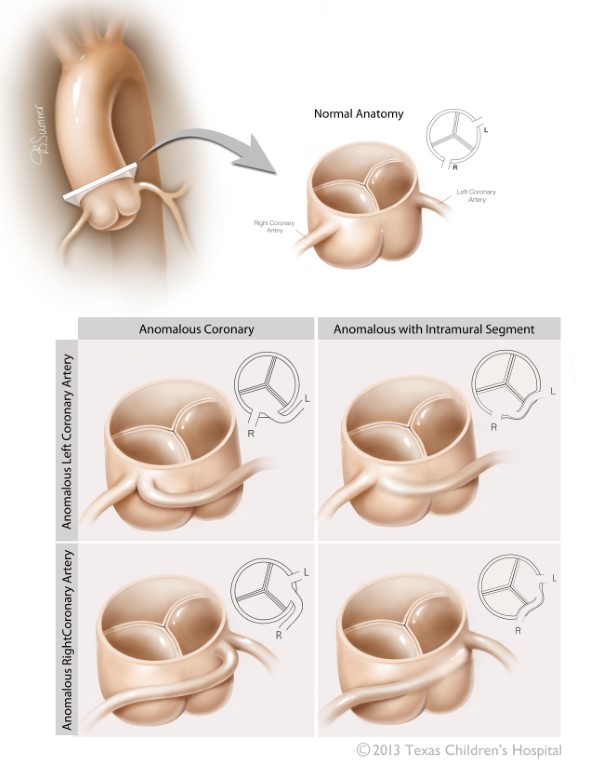
Figure 6 Anomalous Aortic Origin of the Coronary Arteries
The two variants of anomalous origin of the coronary arteries most commonly associated with sudden death are displayed with depiction of their respective intramural segments. L left coronary cusp, R right coronary cusp. Source: Texas Children’s Hospital, with permission.
Although of the two, an anomalous RCA is the most common lesion, an anomalous LMCA is linked to higher mortality. Additionally, these arteries may have an abnormal interarterial or intramural course. The term interarterial indicates that the anomalous vessel courses between the great arteries. An intramural course is when the vessel is embedded in the anterior wall of the aorta between the great vessels and can infer a higher risk of sudden cardiac death. Although several mechanisms have been proposed, it is speculated that ischemic events may result from compression by the great arteries during exertion in the presence of slit like coronary ostia.
Transesophageal Echocardiography
Pre-Operative Assessment
The ME AV SAX and ME RV In-Out views in most cases provide excellent imaging of AAOCA, especially those anomalies with an interarterial or intramural course (Figure 7, Video 3).
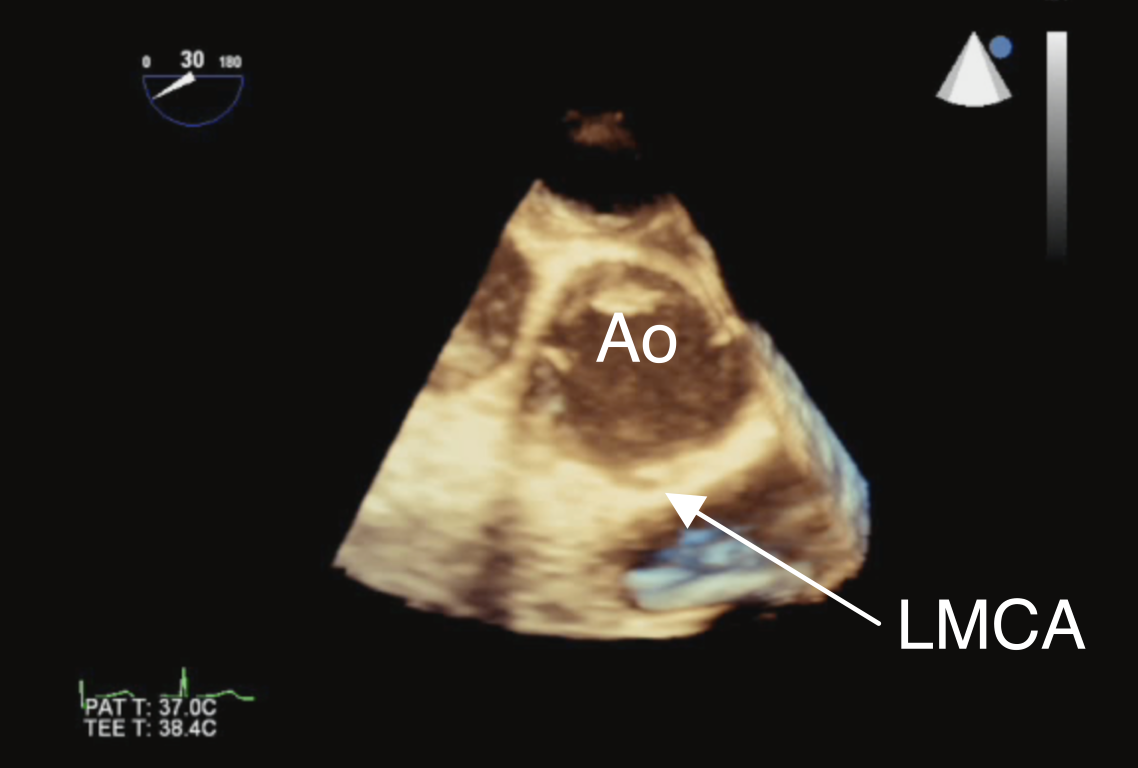
Figure 7 Anomalous Origin of Left Main Coronary Artery from the Right Aortic Sinus
3D TEE view displaying the left main coronary artery (LMCA) as it originates anomalously from the right sinus of Valsalva by three-dimensional TEE imaging. Ao aorta
Video 3 Anomalous Origin of Left Main Coronary Artery from the Right Aortic Sinus
ME AoV SAX 2D image depicting an anomalous left main coronary artery (LMCA) arising from the right aortic sinus near the intercoronary commissure. Note the origin of the right coronary artery (RCA) from the same sinus of Valsalva.
In the case of an intramural course, 2D imaging will capture this segment as an echo lucent linear space along the anterior aortic wall and color Doppler will show an abnormal linear diastolic signal in this region. This signal can also be interrogated by spectral Doppler to confirm the presence of coronary flow, velocity, pattern, and direction (12). Color flow mapping is also useful in distinguishing the type of anomaly by identifying the origin of the coronary artery in question. For example, with an anomalous LMCA from the right sinus of Valsalva, a red color Doppler signal will appear in the anomalous vessel as the flow moves from the right sinus posteriorly, or towards the TEE probe. Conversely, if the RCA arises anomalously from the left sinus of Valsalva, a blue color Doppler signal will be imaged as the flow moves anteriorly in a left to right orientation or away from the TEE transducer (Figure 8, Video 4).
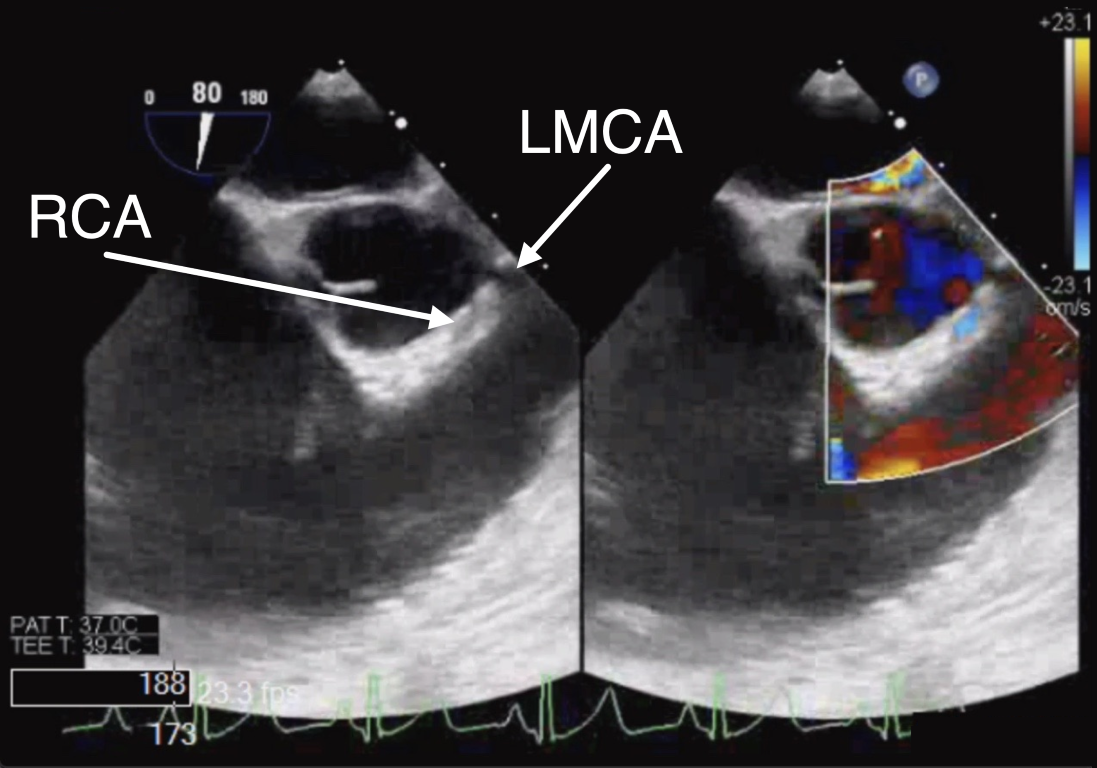
Figure 8 Anomalous Origin of Right Coronary Artery from the Left Aortic Sinus
ME RV In-Out 2D (left panel) and color Doppler (right panel) view of the right coronary artery (RCA) as it originates anomalously from the left coronary cusp. LMCA left main coronary artery
Video 4 Anomalous Origin of Right Coronary Artery from the Left Aortic Sinus
ME RV In-Out 2D and color Doppler images depicting an anomalous right coronary artery (RCA) arising from the left aortic sinus. Note the origin of the left main coronary artery (LMCA) from the same sinus of Valsalva.
The anomalous coronary artery can be imaged running between the great arteries in the ME LAX view as a discrete circle anterior to the aortic root (Figure 9). Counterclockwise rotation of the probe brings the LMCA into view and clockwise rotation of the probe will image the RCA.
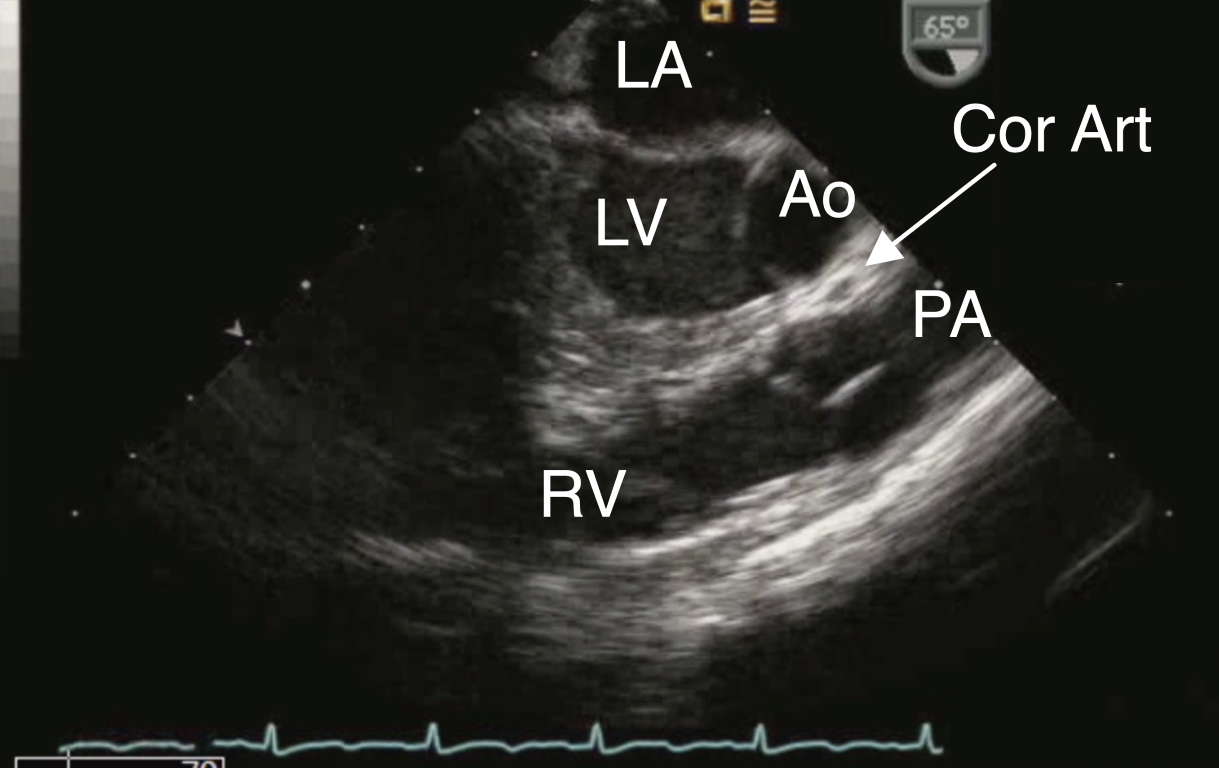
Figure 9 Anomalous Origin of Right Coronary Artery off the Left Sinus of Valsalva
ME LAX view displaying an anomalous coronary artery (Cor Art), seen as a circle anterior to the aorta (Ao). LA left atrium, LV left ventricle, PA pulmonary artery, RV right ventricle
Postoperative Examination
Surgical intervention of an AAOCA usually involves unroofing the intramural segment of the anomalous artery, if present, but in some cases requires reimplantation of the anomalous vessel to the appropriate sinus, patch enlargement of the anomalous coronary origin, or rarely, coronary artery bypass grafting. For the unroofing procedure, the common wall between the aorta and anomalous artery is excised to create a neo-orifice in the correct sinus (Figure 10). This neo-orifice will be perpendicularly oriented to the aorta and can be best characterized in the ME AV SAX view (Video 5).
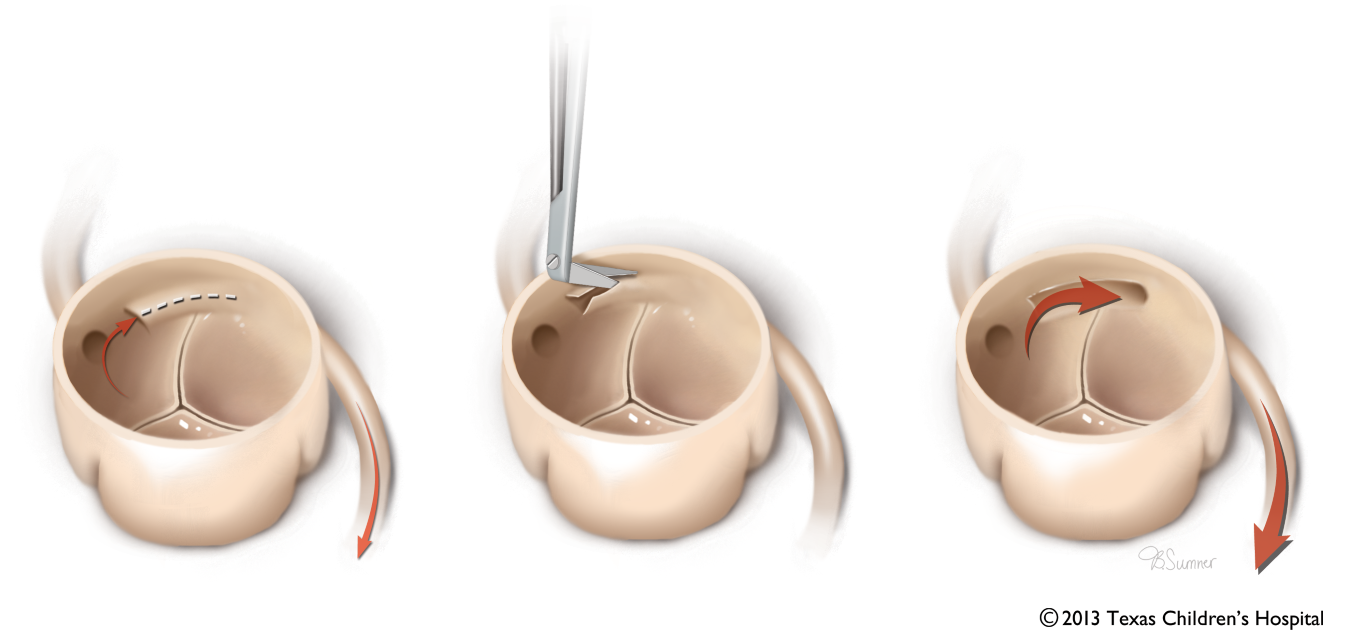
Figure 10 Unroofing Procedure of Intramural Segment in Anomalous Coronary Artery
Depiction of unroofing of the common wall between the aorta and an anomalous coronary artery which allows for creation of a neo coronary orifice in the appropriate sinus. Source: Texas Children’s Hospital, with permission.
Video 5 Unroofed Intramural Segment of Anomalous Coronary Artery
Color flow Doppler interrogation after unroofing procedure of anomalous right coronary artery (RCA) depicting laminar flow.
Color Doppler interrogation should confirm unobstructed coronary artery flow from the appropriate sinus. Determination of spectral Doppler velocities across the unroofed vessel is helpful in the exclusion of flow obstruction. Alternatively, epicardial imaging, also referred to as E-echo, may be used in the post bypass period to assess adequacy of the repair if TEE imaging of the coronary arteries is not feasible or adequate (please refer to the section on Epicardial Echocardiography for further details).
Anomalous origin of the left coronary artery from the pulmonary artery
Anomalous origin of the left coronary artery from the pulmonary artery (ALCAPA) while rare, occurs when the LMCA arises from the pulmonary root (Figure 11). This condition is also known as also known as Bland-White-Garland syndrome. Patients with this anomaly are at significant risk for LV dysfunction related to ischemia and/or infarction, and even death if the condition is left untreated (13). Poor myocardial perfusion in infancy results from coronary steal. This occurs due to the normal reduction in the diastolic pulmonary artery pressure, allowing left coronary artery blood to reflux toward the pulmonary artery instead of the myocardium. Blood supply to the myocardium in the territory of the LMCA is therefore provided by collateral circulation from the RCA. Some patients may develop collateral coronary circulation and present later in life.
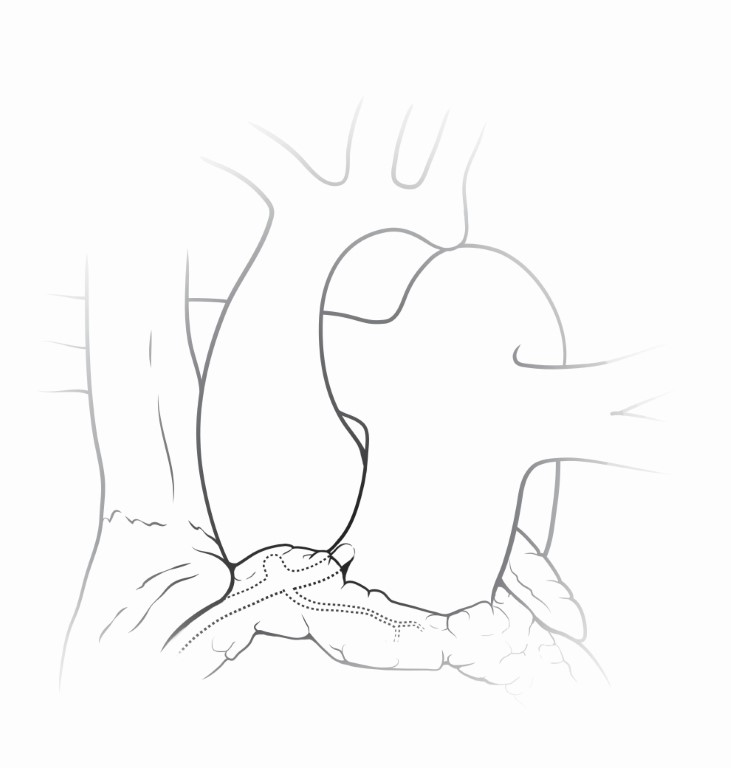
Figure 11 Anomalous Origin of Left Main Coronary Artery from the Pulmonary Artery
The left main coronary artery is depicted in this graphic arising anomalously from the pulmonary root. Patients with this anomaly are at significant risk for LV dysfunction related to ischemia and/or infarction, and even death if the condition is left untreated. Source: Texas Children’s Hospital, used with permission.
Transesophageal Echocardiography
Pre-operative Examination
Although in most cases the diagnosis of this anomaly is made by a combination of criteria such as clinical picture, ECG findings, and transthoracic imaging, in some instances additional confirmatory imaging modalities such as chest tomography (CT) may be necessary. The goal of the preoperative TEE examination in these patients includes confirmation of the diagnosis by characterizing the origin of the anomalous vessel and defining the direction of the anomalous coronary blood flow. Color Doppler may display the direction of blood flow from the RCA through collaterals. Relevant aspects of the exam also include the evaluation of ventricular function and severity of frequently associated mitral regurgitation.
The anomalous LMCA can be seen as it originates from the MPA in the ME AoV SAX and/or the ME RV In-Out view. At times modification of these views may be necessary to delineate the anatomy. The anomalous vessel typically arises from the posterior and leftward aspect of the MPA near the left aortic sinus of Valsalva and can often be confused with a normal origin by color Doppler. As the anomalous vessel is filled via retrograde collateral circulation, a blue color Doppler signal will appear in the LMCA. If the imaging probe is withdrawn to the upper esophagus, the short axis of the ascending aorta and the long axis of the MPA and branch pulmonary arteries can be viewed in the UE PA view (transducer angle ~0°–20°). Color Doppler imaging in any of these views may depict retrograde anomalous coronary artery blood flow (red signal) into the MPA (Figure 12, Video 6).
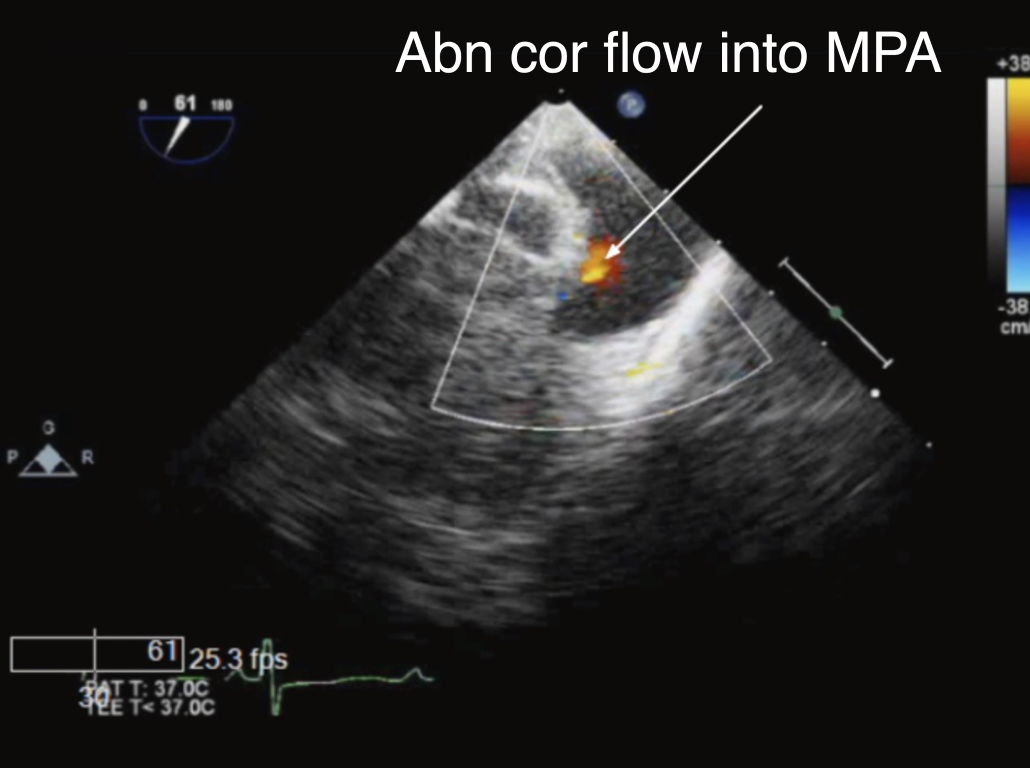
Figure 12 Color Doppler in the Main Pulmonary Artery in Anomalous Origin of Left Main Coronary Artery from the Pulmonary Artery
ME RV In-Out view with color flow mapping demonstrating retrograde abnormal red flow into the main pulmonary artery (MPA) corresponding to an anomalous left coronary artery (arrow).
Video 6 Anomalous Origin of Left Main Coronary Artery from the Pulmonary Artery (ALCAPA)
ME RV In-Out view with color flow Doppler imaging depicting abnormal retrograde flow into the main pulmonary artery (MPA) in diastole from an anomalous left main coronary artery (red color Doppler signal noted by arrow)
The RCA may have a dilated appearance due to the collateral blood flow (Figure 13, Video 7). The baseline assessment of LV function should be performed using the views previously described. The evaluation of mitral regurgitation requires 2D and Doppler interrogation in multiple views that include the ME 4-Ch, ME 2-Ch, ME Mitral, and the ME LAX views (for further discussion of mitral valve assessment the reader is directed to the Mitral Valve Echo Tutorials on this website).
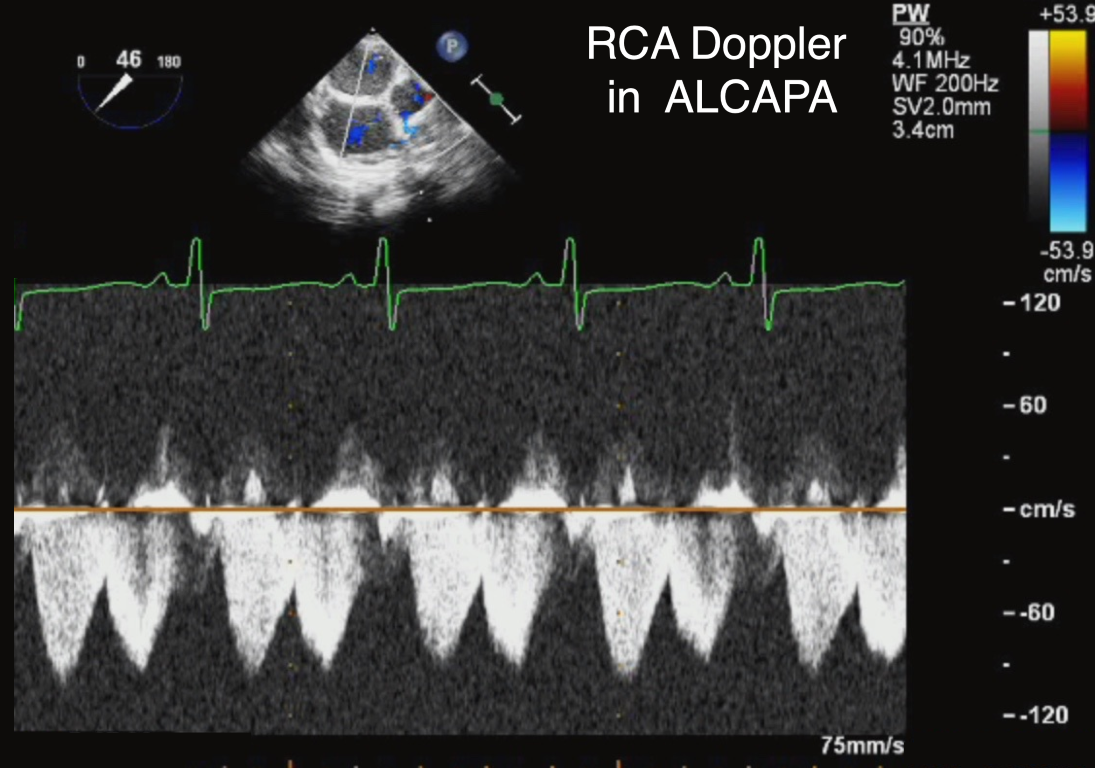
Figure 13 Right Coronary Artery in Anomalous Origin of the Left Coronary Artery from the Pulmonary Artery (ALCAPA)
Modified midesophageal view with pulsed-wave Doppler across the right coronary artery in ALCAPA demonstrating the typical pattern of increased flow across the vessel, consistent with collateral blood supply to the abnormal left coronary artery.
Video 7 Right Coronary Artery in Anomalous Origin of the Left Coronary Artery from the Pulmonary Artery (ALCAPA)
Dilated right coronary artery with prominent flow by color Doppler in ALCAPA as it supplies the anomalous coronary via collateral vessels.
Post-operative Examination
The surgical treatment of this malformation has evolved over time. At the present the favored technique involves reimplantation of the anomalous coronary ostia into the aorta. For this purpose, a button containing the anomalous vessel is usually harvested and translocated into the aortic root while the defect in the PA is repaired. This can be more challenging if the anomalous vessel does not originate from the sinus facing the PA (Figure 14).
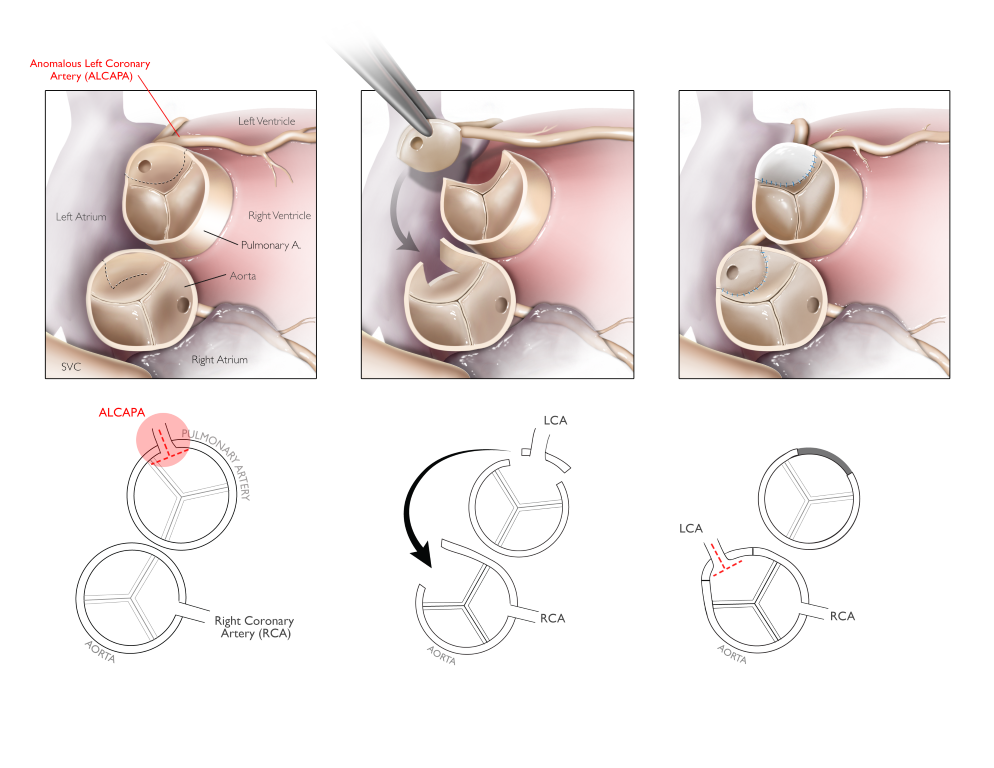
Figure 14 Reimplantation of Anomalous Left Main Coronary Artery into the Aorta
Reimplantation of an anomalous coronary artery originating from the pulmonary artery involves creating a button containing the vessel and translocating it into the aorta while the defect in the pulmonary artery is repaired. The graphic displays a less common variant of this condition where the anomalous coronary originates from a non-facing sinus. Source: Texas Children’s Hospital, with permission.
The Takeuchi procedure represents an alternate technique if this approach is not feasible due to unfavorable anatomy (Figure 15). This involves the creation of an aortopulmonary window and a tunnel inside the PA that baffles blood from the aorta into the left coronary ostium.
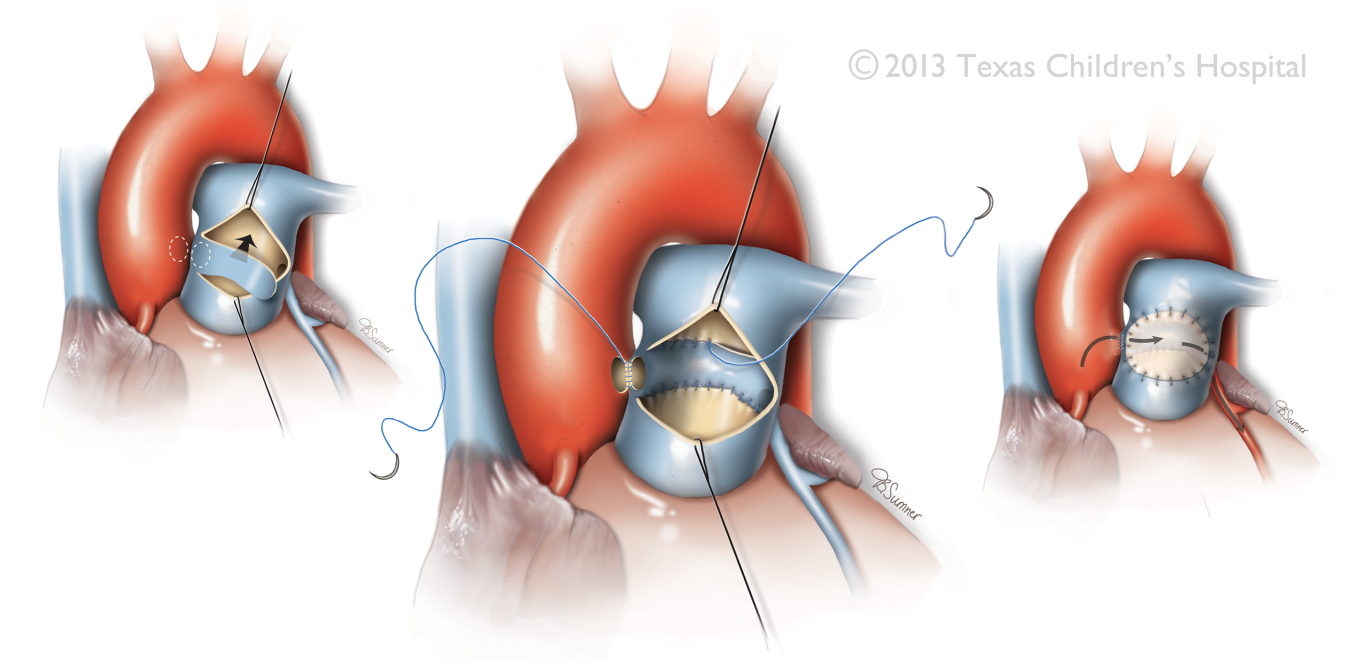
Figure 15 Takeuchi Surgical Procedure
The surgical technique involves creation of an aortopulmonary window and a tunnel inside the pulmonary artery that baffles blood from the aorta into an anomalous coronary ostium. In the graphic displayed an anomalous left main coronary artery is depicted. Source: Texas Children’s Hospital, with permission.
Regardless of technique, TEE may be used to assess the repair in order exclude obstruction to coronary blood flow, and to evaluate relevant parameters such as ventricular function and degree of mitral regurgitation as previously described. In the Takeuchi repair, imaging of the MPA as allowed by the ME RV In-Out, UE PA, and complementary views provides for assessment of potential baffle obstruction or leak, and stenosis across the MPA. The deep transgastric RVOT view can also be helpful in the Doppler interrogation of the pulmonary outflow tract to exclude stenosis given the favorable spectral Doppler alignment allowed by this view.
Kawasaki Disease
Kawasaki disease (KD), also known as mucocutaneous lymph node syndrome, is the most common acquired form of pediatric heart disease in developed countries. The inflammatory process can cause an acute vasculitis syndrome resulting in large vessel arteritis, arterial aneurysms, pericarditis, myocarditis, and aortic root enlargement. The coronary arteries are particularly vulnerable and may develop ectasia, aneurysm, and at times intraluminal thrombus formation, which are the most severe consequences of the disease process (Figure 16) (14). Ischemic heart disease or sudden death may even result from the coronary vasculitis. The affected coronary arteries in decreasing order of incidence are the: proximal LAD and RCA, LMCA, LCx, and distal RCA.
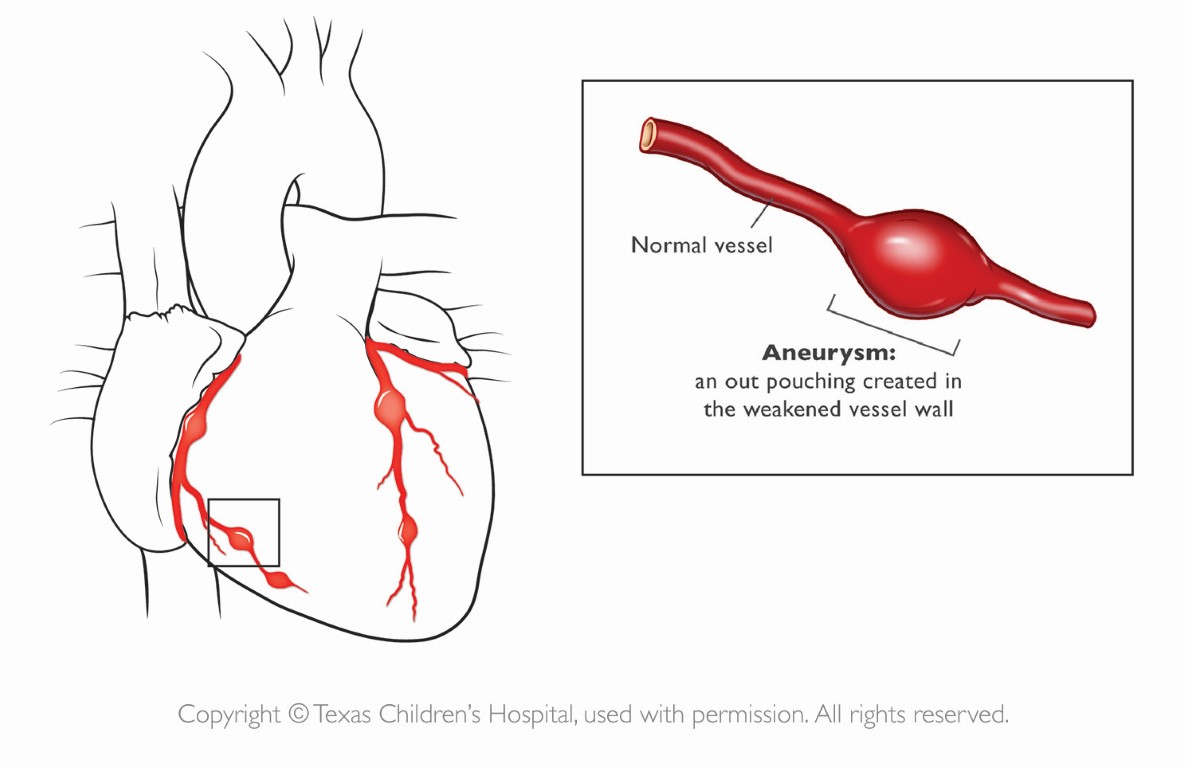
Figure 16 Coronary Artery Aneurysms in Kawasaki Disease
In severe cases, patients with Kawasaki Disease may develop coronary artery aneurysms, the most severe consequence of the disease process, as shown. Source: Texas Children’s Hospital, with permission.
Clinically similar to KD is the newly emerging diagnosis of Multisystem Inflammatory Syndrome in Children (MIS-C). In March 2020 the World Health Organization declared a global pandemic due to the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2). In the months following this declaration, it was soon noticed that a small proportion of children who contracted SARS-CoV-2 were observed to develop multisystem inflammation with features of fever, desquamation, coronary artery aneurysms, and myocardial dysfunction (15).
Transthoracic echocardiography is the preferred imaging modality to document the cardiac manifestations of KD and for serial follow up in affected children. Other modalities commonly used include CT, cardiac magnetic resonance imaging, and in some cases coronary angiography. TEE is largely utilized in these patients for intraoperative monitoring and evaluation of the surgical results when coronary revascularization is undertaken. Surgical management in KD primarily addresses compromised coronary blood flow. An intervention may be considered when stress testing suggests reversible ischemia, there is viable myocardial tissue where the coronary graft is to be implanted, and no coronary artery lesions are present distal to the graft site.
Transesophageal Echocardiography
Pre-operative Examination
The preoperative TEE examination provides confirmation of the presence of the aneurysms as they arise from the left/and or right coronary arteries. The number and location of aneurysms, as well as their dimensions (measured from inner edge to inner edge excluding branch points) and the presence of thrombi should be evaluated. Helpful views for this assessment include the ME AoV SAX, ME RV In-Out, ME LAX, ME 4-Ch, and ME 2-Ch views (Videos 8 and 9).
Video 8 Right Coronary Artery Aneurysm in Kawasaki Disease
ME In-Out view displaying an aneurysmal right coronary artery with intraluminal thrombus in a patient with Kawasaki disease (arrow). Ao aorta, LA left atrium, RA right atrium, RV right ventricle
Video 9 Left Main Coronary Artery Aneurysm in Kawasaki Disease
ME RV In-Outflow view in the same patient displayed in Video 8 showing a large aneurysm involving the left main coronary artery (LMCA). Note the small blue color Doppler jet in the vessel corresponding the coronary flow. Ao aorta, LA left atrium, RA right atrium, RV right ventricle
Other cross-sections (Figures 17 and 18, Video 10) can also be helpful. Most TEE imaging of the coronary arteries is performed at the level of the mid esophagus although large or giant aneurysms can also be evident in the transgastric and deep transgastric windows. Since coronary artery blood flow can be affected related to the pathology described, it is important to bear in mind the myocardial blood flow distribution of each of these vessels and regional functional assessment as previously described.
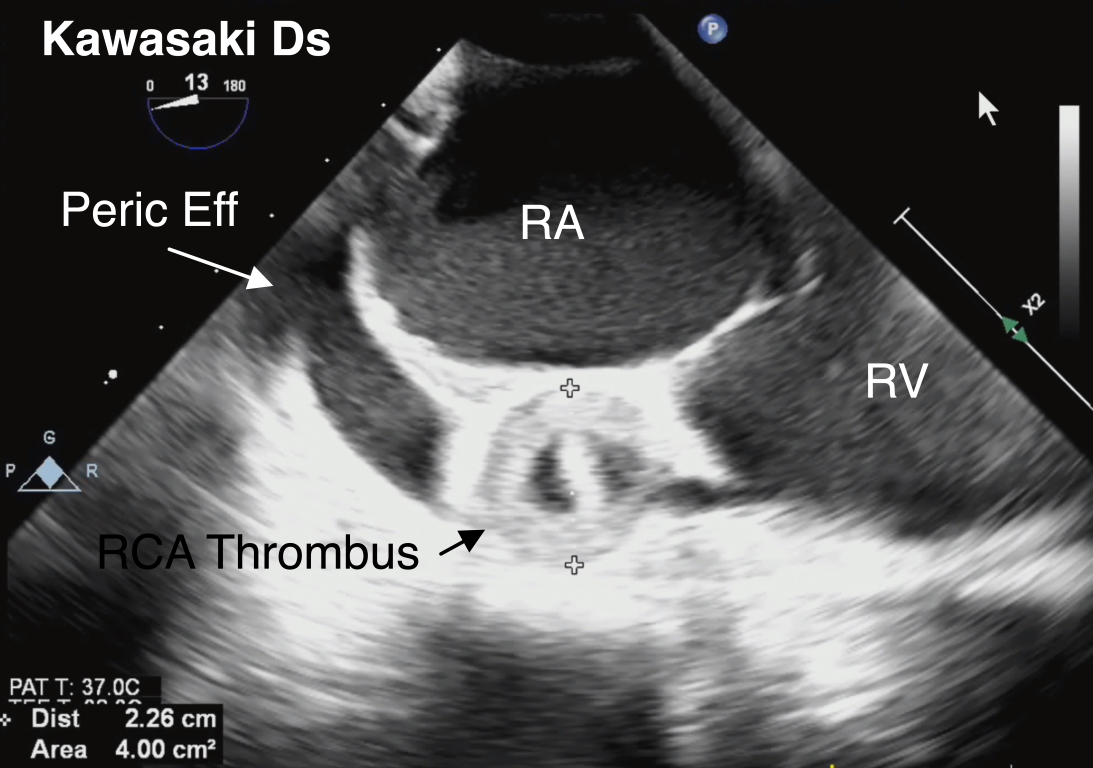
Figure 17 Right Coronary Artery Aneurysm in Kawasaki Disease
A large right coronary artery (RCA) aneurysm measuring 2.2 cm with thrombus formation is identified along the atrioventricular groove in this ME 4-Ch view with rightward-turning of the TEE probe. This is the same patient depicted in Videos 8 and 9). Also shown is a pericardial effusion (peric eff), which represents another cardiac manifestation of Kawasaki Disease. RA right atrium, RV right ventricle
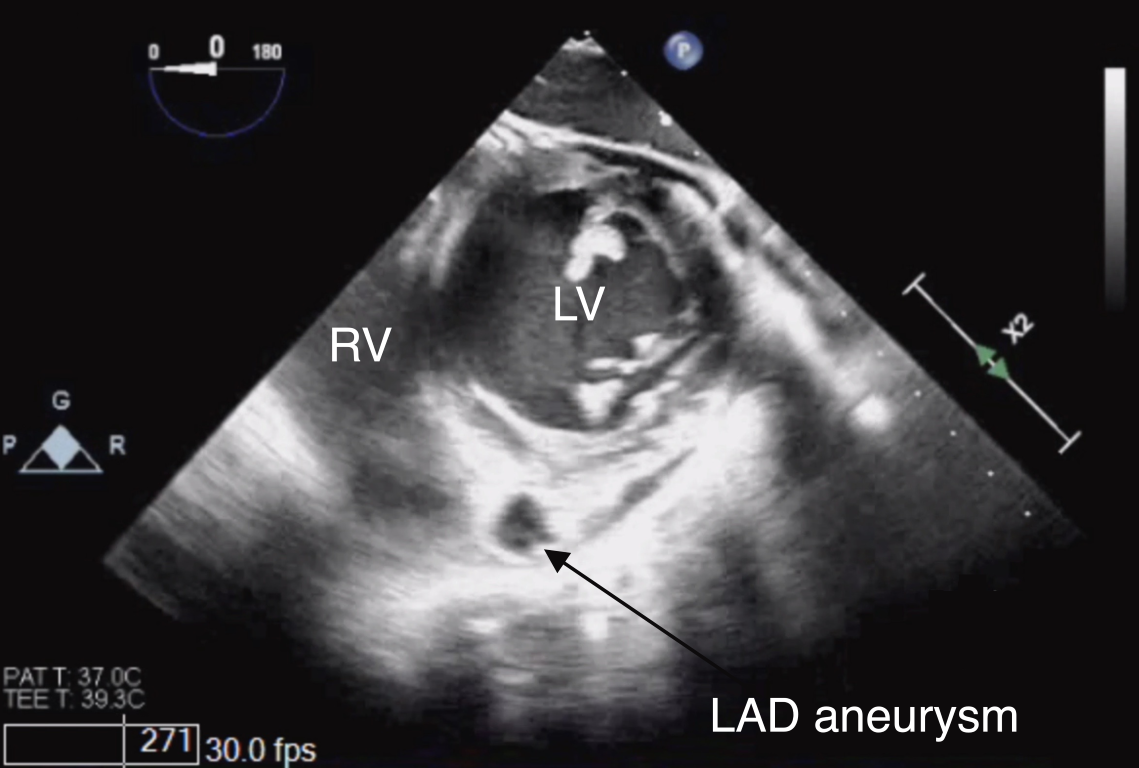
Figure 18 Left Anterior Descending Coronary Artery Aneurysm in Kawasaki Disease
TG Mid PAP SAX view depicting an aneurysm of the left anterior descending coronary artery (LAD) in its short-axis. Note the location of the vessel in the anterior interventricular groove between the right (RV) and left ventricles (LV).
Video 10 Left Anterior Descending Artery Aneurysm in Kawasaki Disease
A large aneurysm of the left anterior descending artery (arrow) is depicted in the Mid PAP SAX View. LV left ventricle, RV right ventricle
As mentioned, other cardiac manifestation of KD include myocarditis, valvulitis, and effusions. In the acute course, myocarditis can manifest as reduced ventricular function which can be examined qualitatively by using the combination of views as outlined. Grading the severity of mitral valvulitis can be assisted by color flow Doppler imaging in multiple views that display the valve in cross-section (refer to prior sections). Mitral valve regurgitation in the context of KD can be due to transient papillary muscle dysfunction or myocardial infarction. This can also be examined in the views previously described for mitral valve evaluation. The presence of pericardial effusions can be determined using a combination of TEE views, but most commonly the examination is initiated in the ME 4-Ch and TG SAX views.
Post-operative Examination
Postoperative TEE should focus on addressing global ventricular function, regional wall motion abnormalities, and mitral regurgitation.
Coronary Artery Reimplantation Procedures
Surgical interventions for CHD such as the arterial switch operation (ASO) for d-transposition of the great arteries (d-TGA), the Ross operation, and others, involve reimplantation of the coronary arteries (Figure 19). This crucial step if done improperly, can lead to inability to wean from cardiopulmonary bypass, the need for high inotropic support, arrhythmias, and impaired ventricular function due to myocardial ischemia (16).
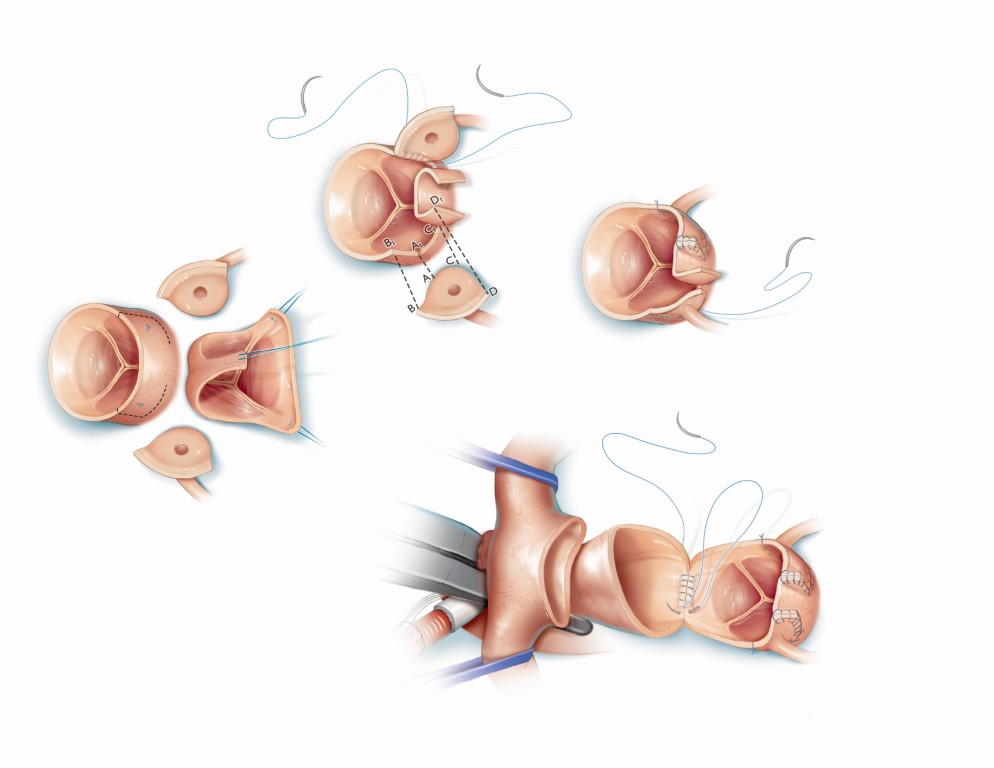
Figure 19 Coronary Artery Reimplantation during the Arterial Switch Operation for d-Transposition of the Great Arteries
Graphic depicts the arterial switch operation during which coronary buttons are harvested on the native aortic root and mobilized to the native pulmonary artery (neoaorta after the procedure). Source: Texas Children’s Hospital, with permission.
The preoperative characterization of coronary anatomy is important given the fact that in some congenital cardiac anomalies specific patterns may infer difficulty with reimplantation. In most cases this evaluation is performed by TTE. Coronary artery imaging may be particularly important in the context of d-TGA, as abnormalities infer the greatest risk for mortality, reoperation, ischemic events, and long-term morbidity. In fact, imaging of the coronary arteries, has been utilized and studied in its ability to predict postoperative morbidity after coronary artery reimplantation (17, 18).
Transesophageal Echocardiography
Preoperative Evaluation
In d-TGA the most common coronary artery variants are: 1) the usual pattern, where the coronary arteries arise from the sinuses facing the PA, 2) origin of the LCx from the RCA, where the LCx courses behind the PA, and 3) a single RCA (19). TTE, as mentioned, is best suited for defining preoperative coronary artery anatomy with this diagnosis (for a more detailed discussion please refer to Echo Rounds addressing d-TGA). TEE is primarily used for confirmation of all the aspects of anatomy, including the coronary arteries, hemodynamic evaluation of associated lesions, functional assessment, and intraoperative monitoring.
Focused preoperative coronary imaging may not be considered an essential aspect of the TEE examination in patients undergoing surgical procedures where the coronary vessels are normal in origin and proximal course and they are to be reimplanted during aortic reconstruction (e.g., Ross procedure, aortic root replacement). However, the preoperative TEE assessment of the coronary arteries provides a baseline reference for postoperative evaluation after these have been manipulated. As in other patients, TEE provides for characterization and confirmation of the structural abnormalities and baseline assessment of ventricular function.
Postoperative Evaluation
The main goal of the study post ASO is to evaluate the patency of outflow tracts, atrioventricular and semilunar valve function, biventricular systolic function (including regional wall motion), and coronary artery patency. In order to assess the reimplanted coronary arteries, a combination of views at the mid and upper esophagus can be used. The ME AoV SAX and ME RV In-Out views represent a starting point. The ME 5-Ch can also be helpful. The reimplanted vessels may be high in the aortic sinuses and modified planes and views may be necessary or epicardial imaging may be used to complement the exam.
Doppler interrogation, both spectral and color flow, across the main coronary arteries represents an important component of the post bypass TEE examination (Figure 20, Video 11). Pulsed-wave Doppler flow patterns have been studied in their ability to predict post coronary artery reimplantation morbidity associated with ASO (17, 18). An ECG tracing is essential for timing of the coronary artery pulsed-wave Doppler patterns and interpretation of the wave forms. Systolic coronary flow occurs at the time interval from the onset of the QRS wave to the end of the T wave; diastolic flow is defined from the end of the T wave to the onset of the next QRS complex. A biphasic pulsed-wave Doppler pattern in systole and diastole implies unobstructed blood flow (Figure 21). The flow pattern is characterized by a late systolic peak, followed by a lower velocity throughout diastole (4). Median velocity time integrals for the RCA, LCA, and LCx have been reported as 0.112, 0.117, and 0.073 respectively; with median peak diastolic flow velocities of 61 cm/s for the RCA, and 40 cm/s for the LCA/LCx (18).
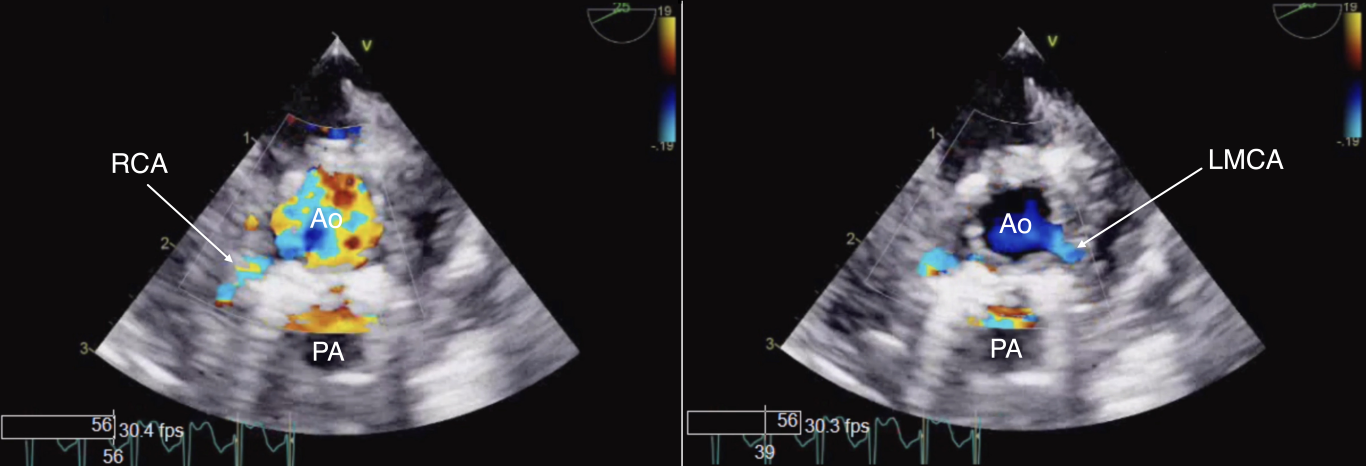
Figure 20 Color Doppler Examination of Reimplanted Coronary Arteries
To assess the reimplanted coronary arteries after an arterial switch operation a combination of TEE views at the mid and upper esophageal levels can be used. The ME AoV SAX view depicted demonstrates color flow interrogation in a reimplanted right coronary artery (RCA, left panel) and left main coronary artery (LMCA, right panel). Ao aorta, PA pulmonary artery
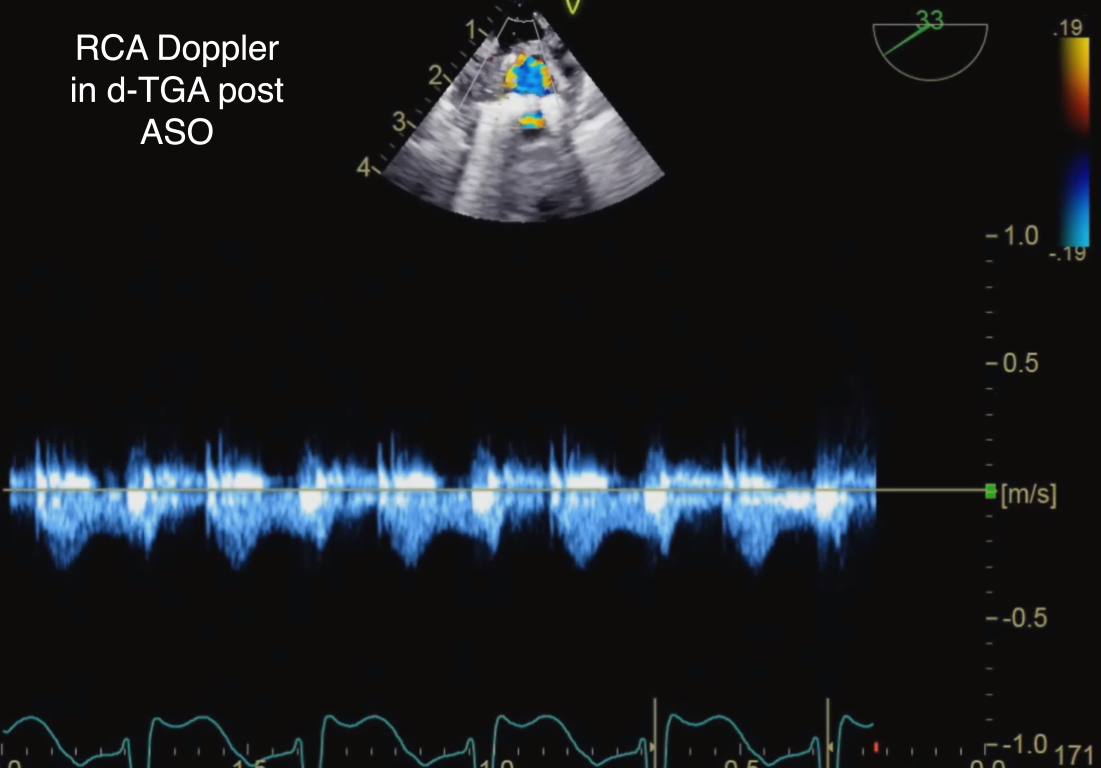
Figure 21 Pulsed-Wave Doppler of Reimplanted Right Coronary Artery (RCA) After Arterial Switch Operation (ASO) in d-Transposition of the Great Arteries (d-TGA)
A low-velocity biphasic flow pattern is depicted across reimplanted right coronary artery after arterial switch operation consistent with unobstructed flow.
Video 11 Color Doppler Interrogation of Reimplanted Coronary Arteries Post Arterial Switch Operation (ASO) in d-Transposition of the Great Arteries (d-TGA)
ME AoV SAX view demonstrates color flow Doppler interrogation of reimplanted coronary arteries after arterial switch operation. Ao aorta, LMCA left main coronary artery, RCA right coronary artery
As mentioned, a laminar antegrade systolic and diastolic color flow signal at low velocities in the coronary arteries indicates a normal flow pattern, whereas aliasing at the standard velocity, or a higher color scale requirement suggests coronary artery narrowing (18). Retrograde flow in the LCA as detected by pulsed-wave Doppler has been strongly associated with postoperative morbidity (18). Higher peak velocities (> 100 cm/s), especially that of the RCA, has been linked to perioperative myocardial events.
As in all procedures that involve coronary interventions, global and regional ventricular function should be assessed as well as atrioventricular valve competency as a potential indicator of ischemia. Regional wall motion abnormalities after the ASO may be the result of a stunned myocardium or myocardial injury during the course of the operation. The feasibility of performing segmental wall motion analysis in this patient population has been reported along with the incremental value of multiple cross-sections in this evaluation (20). However, it is recognized that this assessment is subjective and dependent on the expertise of the imager. Newer TEE imaging approaches, such as strain imaging, may be able to assist in this assessment but as of yet are not practical in the operating room setting.
Epicardial Echocardiography (E-echo)
Epicardial imaging of the coronary arteries can complement the TEE evaluation and, in some cases, offer the only imaging option including at times superior image quality. The approach consists of placement of a transthoracic imaging probe covered with a sterile sheath directly onto the aortic root to obtain a short-axis view of the aortic valve and root, allowing for imaging of both the right and left coronary ostia and proximal course. Alternatively, other techniques described to image the coronary arteries include: 1) placing the probe on the ascending aorta and angling the transducer inferiorly, 2) placing the probe in the LV outflow tract and angling upward, 3) or imaging through the right ventricular outflow tract in the short axis (4, 21). The aortic root may then be imaged in short axis with the RCA viewed at a 10 o’clock position and the LMCA at 2 o’clock. E-echo may be used, similarly to TEE, to confirm coronary artery flow from the appropriate sinus by color and spectral Doppler (Figure 22, Video 12).
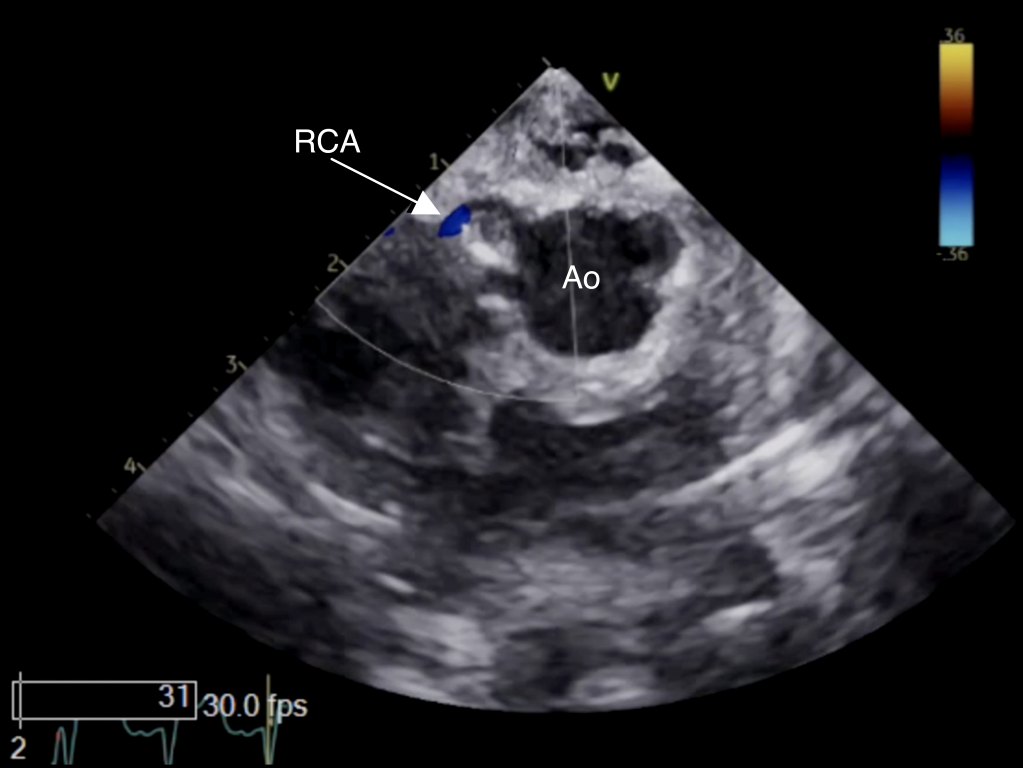
Figure 22 Epicardial Echocardiography of Reimplanted Right Coronary Artery
Epicardial image displaying antegrade flow (arrow) across reimplanted right coronary artery (RCA) after arterial switch operation by color Doppler. Ao aorta
Video 12 Epicardial Echocardiography (E-echo) of Reimplanted Right Coronary Artery
Epicardial image (equivalent to transthoracic aortic valve short-axis view) displaying laminar antegrade flow (arrow) across right coronary artery (RCA)) after arterial switch operation. Ao aorta
Similar to TEE, E-echo has been investigated in its ability to predict outcome after coronary artery reimplantation. E-echo Doppler patterns have been studied and found to be identical to that of TEE (18). Coronary artery pulsed-wave Doppler velocities > 100 cm/s have been suggestive of proximal coronary artery stenosis. The stenosis can be the result of improperly sewing the coronary button leading to vessel kinking. When assessing the reimplanted LCA, there are certain findings associated with adverse myocardial events: 1) retrograde blood flow on the reimplanted vessel by pulsed-wave Doppler, 2) velocity time integral in the coronary artery > 0.14, and 3) peak systolic velocity in the LMCA > 60 cm/s (17).
While E-echo has its proven advantages, in terms of its ability to image coronary arteries, it is not without limitations. It can be associated with potential hemodynamic compromise, arrhythmias, and infection. The technique also requires a second operator of the echocardiographic system, expertise in epicardial imaging by the surgeon, and for the surgical procedure to pause while imaging in undertaken.
Conclusion
Anomalies of the coronary arteries are rare and may be congenital or acquired in nature. Regardless of etiology, intraoperative imaging is of benefit for confirmation of coronary anatomy and for guiding medical and surgical therapies. TEE is able to characterize associated defects, examine hemodynamics, and capture related repercussions of coronary insufficiency such as ventricular dysfunction (global and segmental) or valvar regurgitation. TEE also plays an important role in intraoperative monitoring and determining of the adequacy of the surgical intervention. In patients who have undergone procedures involving coronary artery manipulations, TEE is instrumental in the evaluation of coronary blood flow and ventricular function. This imaging modality is particularly useful in cases when there are difficulties during separation from cardiopulmonary bypass in determining possible etiologies.
The various modalities of echocardiography each have their strengths and limitations in terms of their unique ability to image the coronary arteries. TEE is the intraoperative imaging modality of choice and E-echo in most cases complements this assessment. As the technology continues to evolve facilitating the acquisition and interpretation of intraoperative imaging data, current challenges may be overcome. It should be emphasized that these intraoperative imaging approaches provide significant benefits in the evaluation of congenital and acquired coronary anomalies. However, they may not be able to definitively exclude compromised coronary flow in some cases. As such, if clinically important concerns remain regarding coronary patency, alternative imaging modalities (chest tomography or cardiac angiography) should be strongly considered in all cases.
Table I
Coronary Arteries (Course, Myocardial Region Supplied, and Branches)
| Left Main or Left Coronary Artery (LMCA or LCA) |
| Course: between the RVOT (anteriorly) and the LA (posteriorly)
Branches: left anterior descending (LAD) and left circumflex (LCx) coronary arteries; a third artery may be present referred to as the ramus intermedius, which has variable branching
Left anterior descending artery Course: along the anterior interventricular groove continuing to the cardiac apex Supplies: anterior, anterolateral, and anteroseptal walls of LV |
| Branches: septal perforators, diagonal arteries |
| Circumflex coronary artery
Course: travels along the left AV groove between the LA and LV towards the crux (where the AV groove intersects with IVS Supplies: lateral LV wall |
| Branches: obtuse marginal arteries, posterior descending artery (if left dominant coronary system*) |
| Right Coronary Artery (RCA) |
| Course: along the right AV groove between the RA and RV
Supplies: RA, SA node, AV node, RV, inferior LV wall, inferior ventricular septum |
| Branches: conal branch, SA node artery, acute marginal arteries, posterior descending artery and posterior LV branch (if right dominant coronary system*) |
Abbreviations: AV atrioventricular, IVS interventricular septum, LA left atrium, LV left ventricle, RA right atrium, RV right ventricle, RVOT right ventricular outflow tract, SA sinoatrial
*The artery that supplies the posterior descending artery and the posterolateral branch determines coronary dominance. If these arise from the RCA the system is considered to be right dominant (80–85% of cases).
Table II
Main TEE Views for Visualization of the Coronary Arteries
| TEE View (Abbreviation) | Transducer Angle | Structures Visualized |
| Midesophageal Aortic Valve Short-Axis
(ME AoV SAX) |
~25°–45° | · AoV, LA, RA, IAS, RV, RVOT, PV
· To image the origins and proximal coronary arteries: scan superior to the aortic valve o RCA: transducer angle ~0°–20° (vessel appears at ~ 7 o’clock position) o LMCA: transducer angle ~30°–40° (vessel appears at ~ 2 o’clock position) |
| Midesophageal Aortic Valve Long-Axis
(ME AoV LAX) |
~120°–140° | · LA, MV, LV, LVOT, RVOT, AoV, Prox Asc Ao
· To image the LMCA bifurcating into the LAD and LCx: apply leftward rotation of the probe shaft · To image the mid aspect of the RCA: apply rightward rotation to the probe shaft |
| Midesophageal Right Ventricular Inflow-Outflow
(ME RV In-Out) |
~50°–70° | · AoV, LA, RA, IAS, TV, RV, RVOT, PV, IVS
· To image the mid aspect of the RCA follow the proximal RCA starting from the ME AoV SAX view and adjust transducer angle |
| Midesophageal Five-Chamber
(ME 5-Ch) |
~0°–10° | · AoV, LA, MV, LV, LVOT, RA, TV, RV, IVS
· To image the origins and immediate proximal course of the RCA and LMCA: start with the probe in neutral position in the mid esophagus and once the midesophageal four-chamber view is displayed, anteflex and/or slightly withdraw the probe |
Abbreviations: AoV aortic valve, IAS interatrial septum, IVS interventricular septum, LA left atrium, LCx circumflex coronary artery, LAD left anterior descending coronary artery, LMCA left main coronary artery, LV left ventricle, LVOT left ventricular outflow tract, MV mitral valve, PV pulmonary valve, RA right atrium, RCA right coronary artery, RV right ventricle, RVOT right ventricular outflow tract, TV tricuspid valve
Table III
Main TEE Views for Global and Regional LV Functional Assessment
| TEE View | Coronary Artery | Segment Supplied by Coronary Artery* |
| Midesophageal Four-Chamber
(ME 4-Ch) |
RCA | RV free wall and mid and basal inferoseptum |
| LAD
|
Basal anterolateral and mid anterolateral walls; apical lateral, apical septum, and apical cap | |
| LCx | Basal anterolateral, mid anterolateral, apical lateral walls | |
| Midesophageal Two-Chamber
(ME 2-Ch) |
RCA | Basal and mid inferolateral walls |
| LAD | Mid anterior, basal anterior, apical anterior apical inferior, apical cap | |
| Midesophageal Long-Axis
(ME LAX) |
RCA | Basal and mid inferolateral walls |
| LAD
|
Mid anteroseptum, basal anteroseptum apical anterior, apical lateral, apical cap | |
| Cx | Basal and mid inferolateral walls | |
| Transgastric Short-Axis
(TG SAX) |
RCA
|
Inferior and inferolateral walls; inferoseptum, posteromedial papillary muscle |
| LAD
|
Mid and basal anteroseptum; apical anterior, apical lateral and apical cap; anterolateral papillary muscle | |
| LCx | Anterolateral and inferolateral walls; anterolateral papillary muscle |
* Note that variability may exist in the blood supply to the various myocardial segments.
Abbreviations: RCA right coronary artery, RV right ventricular, LAD left anterior descending coronary artery, LCx left circumflex coronary artery.
References
- Mavroudis C, Dodge-Khatami A, Stewart RD, Jacobs ML, Backer CL, Lorber RE. An overview of surgery options for congenital coronary artery anomalies. Future Cardiol. 2010;6:627-645.
- Twite MD, Stenquist S, Ing RJ. Williams Syndrome. Paediatr Anaesth. 2019;29:483-490.
- Puchalski MD, Lui GK, Miller-Hance WC et al. Guidelines for performing a comprehensive transesophageal echocardiographic examination in children and all patients with congenital heart disease: Recommendations from the American Society of Echocardiography. J Am Soc Echocardiogr. 2019;32:173-215.
- Yim D, Mertens L, Honjo O, Nield LE. Intraoperative echocardiographic coronary artery imaging in congenital and acquired heart disease. Cardiol Young. 2020;30:153-161.
- Chengode S. Left ventricular global systolic function assessment by echocardiography. Ann Card Anaesth. 2016;19:S26-S34.
- Blondheim DS, Beeri R, Feinberg MS et al. Reliability of visual assessment of global and segmental left ventricular function: a multicenter study by the Israeli Echocardiography Research Group. J Am Soc Echocardiogr. 2010;23:258-264.
- Pérez-Pomares JM, de la Pompa JL, Franco D et al. Congenital coronary artery anomalies: a bridge from embryology to anatomy and pathophysiology–a position statement of the development, anatomy, and pathology ESC Working Group. Cardiovasc Res. 2016;109:204-216.
- Bianco F, Colaneri M, Bucciarelli V et al. Echocardiographic screening for the anomalous aortic origin of coronary arteries. Open Heart. 2021;8:e001495.
- Molossi S, Sachdeva S. Anomalous coronary arteries: what is known and what still remains to be learned. Curr Opin Cardiol. 2020;35:42-51.
- Dodge-Khatami A, Mavroudis C, Backer CL. Congenital Heart Surgery Nomenclature and Database Project: Anomalies of the coronary arteries. Ann Thorac Surg. 2000;69:S270-97.
- Brothers JA, Frommelt MA, Jaquiss RDB, Myerburg RJ, Fraser CD, Tweddell JS. Expert consensus guidelines: Anomalous aortic origin of a coronary artery. J Thorac Cardiovasc Surg. 2017;153:1440-1457.
- Frommelt P, Lopez L, Dimas VV et al. Recommendations for Multimodality Assessment of Congenital Coronary Anomalies: A Guide from the American Society of Echocardiography: Developed in Collaboration with the Society for Cardiovascular Angiography and Interventions, Japanese Society of Echocardiography, and Society for Cardiovascular Magnetic Resonance. J Am Soc Echocardiogr. 2020;33:259-294.
- Dodge-Khatami A, Mavroudis C, Backer CL. Anomalous origin of the left coronary artery from the pulmonary artery: collective review of surgical therapy. Ann Thorac Surg. 2002;74:946-955.
- McCrindle BW, Rowley AH, Newburger JW et al. Diagnosis, treatment, and long-term management of Kawasaki disease: A scientific statement for health professionals from the American Heart Association. Circulation. 2017;135:e927-e999.
- Feldstein LR, Rose EB, Horwitz SM et al. Multisystem Inflammatory Syndrome in U.S. Children and Adolescents. N Engl J Med. 2020;383:334-346.
- Fundora MP, Aregullin EO, Wernovsky G et al. Echocardiographic and Surgical Correlation of Coronary Artery Patterns in Transposition of the Great Arteries. Congenit Heart Dis. 2016;11:570-577.
- Wong D, Golding F, Hess L et al. Intraoperative coronary artery pulse Doppler patterns in patients with complete transposition of the great arteries undergoing the arterial switch operation. Am Heart J. 2008;156:466-472.
- Nield LE, Dragulescu A, MacColl C et al. Coronary artery Doppler patterns are associated with clinical outcomes post-arterial switch operation for transposition of the great arteries. Eur Heart J Cardiovasc Imaging. 2018;19:461-468.
- Pasquini L, Sanders SP, Parness IA, Colan SD. Diagnosis of coronary artery anatomy by two-dimensional echocardiography in patients with transposition of the great arteries. Circulation. 1987;75:557-564.
- Rouine-Rapp K, Rouillard KP, Miller-Hance W et al. Segmental wall-motion abnormalities after an arterial switch operation indicate ischemia. Anesth Analg. 2006;103:1139-1146.
- Stern KWD, Emani SM, Peek GJ, Geva T, Kutty S. Epicardial echocardiography in pediatric and congenital heart surgery. World J Pediatr Congenit Heart Surg. 2019;10:343-350.
- Hahn RT, Abraham T, Adams MS et al. Guidelines for performing a comprehensive transesophageal echocardiographic examination: Recommendations from the American Society of Echocardiography and the Society of Cardiovascular Anesthesiologists. J Am Soc Echocardiogr. 2013;26:921-964.
