Contributors
Raquel G. Hernandez, DO and Denise Joffe, MD
Seattle Children’s Hospital
Objectives:
This manuscript describes the transesophageal echocardiographic findings of selected congenital lesions of the mitral valve (MV).
Please see part 1 of this contribution for a review of the comprehensive transesophageal echocardiographic examination of the MV. Refer to table 1 of that document for an outline of relevant aspects of the examination in a patient with MV disease.
Congenital Mitral Stenosis
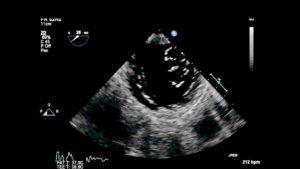
Congenital mitral stenosis (MS) rarely occurs in isolation. The incidence is about 0.6% of autopsied patients with congenital heart disease (CHD) and 0.21% to 0.42% of clinical cases of CHD.1,2 It coexists with a ventricular septal defect (VSD) in about 30% of cases, but is most commonly associated with left heart underdevelopment, left ventricular outflow tract obstruction (LVOTO), and Shone’s complex.1, 2 Shone’s complex is a rare malformation comprised of four left-sided anomalies: a supravalvar mitral membrane (also called a supramitral ring), a parachute MV, subaortic stenosis, and coarctation of the aorta. A double orifice MV, named for the anatomical abnormality it produces, is also an extremely rare congenital anomaly that can lead to either MS or mitral regurgitation (MR). (Figure 1, Video 1)
Figure 1– Double orifice mitral valve imaged from a TG Basal SAX view. Two separate mitral valve orifices are seen in diastole.
Video 1– TG Basal SAX view shows a double orifice MV
Congenital MS is classified into 4 types by a description of the valve abnormality although several types share common features such as short thick chords, obliteration of interchordal spaces and abnormal papillary muscles. 3 (Figure 2)
Figure 2– Typical appearance of abnormal chords in congenital MS. Note the short chordae tendineae with partial or complete obliteration of interchordal spaces.
Ruckman and Van Praagh’s classification: 3
• Typical MS (type I)
◦ thickened and rolled leaflets
◦ short chordae tendineae
◦ partial or complete obliteration of interchordal spaces
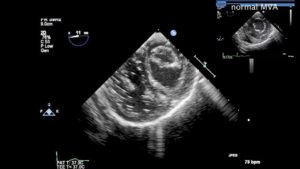
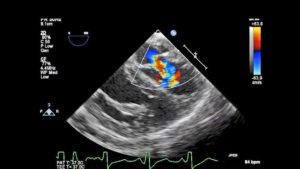
◦ underdeveloped papillary muscles and commissural fusion (Figures 3 and 4, Video 2)
Figure 3– TG Basal SAX view in diastole demonstrating limited opening of the MV, and thickened leaflets. For comparison, the image in the inset shows a normal MV in the same imaging plane.
Figure 4– ME 4C view in diastole with color flow Doppler showing a proximal isovelocity surface area (PISA) forming across the MV (see part 1 of the review for a description of PISA). The valve leaflets appear thickened.
Video 2– ME 4C view with color flow Doppler showing congenital MS with high velocity mitral inflow and PISA formation. Note the thick leaflets and the short restricted posterior leaflet which appears tethered to the papillary muscle. A TG Basal SAX view shows the restricted opening in diastole compared to the inset which demonstrates a normal MV.
• Hypoplastic congenital MS (type II)
◦ small MV orifice
◦ shortened chordae tendineae
◦ small papillary muscles
• Supravalvar MS (type III)
◦ circumferential ridge of connective tissue from LA wall overlying MV leaflets
◦ variability in thickness
◦ attached to the annulus
◦ impairs leaflet mobility (Figures 5 and 6, Video 3)
Figure 5– Modified ME 4C view in diastole. The frame is obtained during diastole when the mitral leaflets open. A supra-mitral ring (membrane) is seen between the two open leaflets.
Figure 6– ME 2C view with color flow Doppler in diastole. A PISA envelope is forming above the annulus consistent with a supramitral ring.
Video 3– ME 2C view showing high velocity mitral inflow and PISA formation above the MV leaflets is consistent with a supramitral ring. A ME modified 5C view demonstrates the membrane above the annulus.
• Parachute mitral valve (type IV)
◦ presence of the usual two leaflets and commissures
◦ all chordae tendineae merged onto one papillary muscle instead of two papillary muscles, creating a parachute-like appearance of the MV apparatus. (Figure 7, Video 4)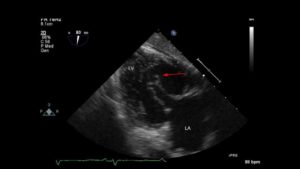
Figure 7- TG 2C view of the left ventricle showing a typical parachute-like appearance of the MV. Other views (not shown) demonstrated the single papillary muscle.
Video 4– TG 2C and ME AV LAX views show the typical funnel-shaped appearance of a parachute MV. Note that the long redundant chords are an additional abnormality in this patient but not typical of a parachute MV.
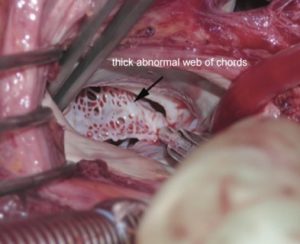
• Hammock mitral valve (also considered MS type IV)
◦ dysplastic with shortened chordae directly inserted into posterior wall of the left ventricle (LV)
◦ tethering of both leaflets
◦ valvar orifice is partially obstructed by a web of chordae
◦ chordae tendinae of anterior leaflet cross orifice toward posteriorly implanted papillary muscle creating hammock appearance
Congenital Mitral Regurgitation:
Isolated severe mitral regurgitation (MR) is rare in pediatric patients. Etiologies include primary problems with the MV such as a cleft, also referred to as zone of apposition, when a component of an atrioventricular septal (AVSD) or canal defect (AVC), or mitral valve prolapse (MVP). Secondary or functional MR usually occurs in the setting of a cardiomyopathy or in combination with ventricular dysfunction. In this setting, the valve itself is usually normal but the coaptation area is decreased as the annulus dilates as a result of a primary myocardial abnormality.
Mitral Valve Prolapse affects 2-3% of the general population and is a common cause of MR 4 , although less common in children. There is abnormal systolic displacement of one or both leaflets into the left atrium past the mitral annular plane due to disruption or elongation of leaflets, chordae, or papillary muscles.4 MVP may be associated with Marfan syndrome or other connective tissue diseases. (Figure 8, Video 5)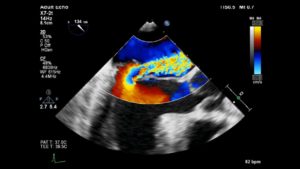
Figure 8– ME AV LAX view showing a prolapsed posterior leaflet with severe MR. The tip of the posterior leaflet is above the annular plane. Note the formation of a PISA below the regurgitant orifice suggesting significant regurgitation.
Video 5– A live 3D exam of the MV looking in an antero-lateral to postero-medial orientation shows bileaflet prolapse. The annulus is marked with an asterisk. A ME commissural view shows P3 prolapse with the leaflet tip above the annular plane. A ME AV LAX view with color flow Doppler shows posterior leaflet prolapse with an anteriorly directed jet of MR.
Cleft Mitral Valve:
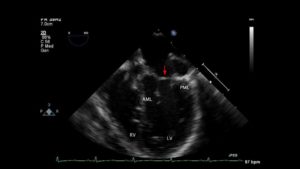
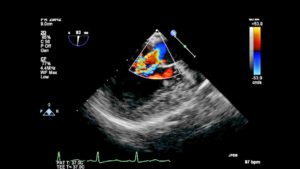
The most common etiology of the ‘so called’ MV cleft is abnormal development of the leaflets in an AVC defect. In normal development, there is fusion of the medial portion of the superior and inferior bridging leaflets at the crux of the heart. Failure of fusion, results in a mitral cleft. The cleft is in the center of the valve where the leaflets failed to fuse or the zone of apposition between the LV components of the bridging leaflets. This is in contrast to other clefts which really are exaggerated scallops that extend deep into the annulus. (Figures 9-11, Video 6).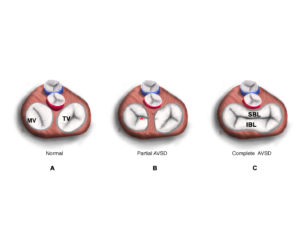
Figure 9 A-C. A. During normal development, the superior and inferior bridging leaflets of the common atrioventricular valves (AV) join to form the anterior leaflet of the MV and the septal leaflet of the TV. B. When there is formation of separate valve orifices but incomplete development of the anterior mitral leaflet then a cleft MV remains (marked with red asterisk). C. The atrioventricular valve in a patient with a complete AVC.
Figure 10 A , B
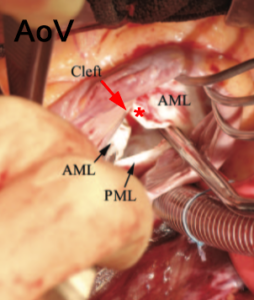
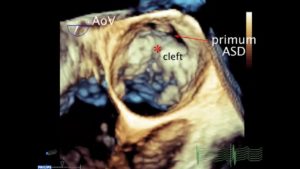
Figure 10 A. Intraoperative photo of a cleft associated with a partial AVC or primum atrial septal defect (ASD). Note the cleft (arrow) at the center of the valve pointing towards the ventricular septum. This is usually the case in AVSDs B. 3D Focused exam of the MV in a patient with a partial AVC defect in diastole. An asterisk marks the cleft in the anterior leaflet. The primum ASD is also seen.
Figure 11– Focused 3D exam of MV in diastole (left panel) and systole (right panel) in a patient with an isolated cleft. The cleft is pointed anteriorly, towards the LV outflow tract and away from the ventricular septum in this case.
Video 6– A focused 3D exam of the MV in a patient with a partial AVC. The image is oriented toward the interventricular septum and the anterior mitral leaflet (AML). A complete cleft in the anterior leaflet is visible. A primum ASD is also seen. A TG basal SAX MV view shows a “V” shaped cleft. A ME AV LAX view shows the cleft in the AML. A focused 3D exam of the MV shows a non AVC-type cleft. The cleft is directed towards the aortic valve.
Miscellaneous Abnormalities of the Mitral Valve:
Abnormal function of the MV apparatus can occur in conditions such as dilated and hypertrophic cardiomyopathy (HOCM), because of abnormalities in the location, size, and/or shape of the papillary muscles.
As described earlier, MR in the setting of a dilated cardiomyoapthy is referred to as functional MR. In these patients, the ventricle dilate and displace the papillary muscles laterally. The annulus also dilates, the chords become tethered, and pull the leaflets apart which decreases the coaptation point and results in MR. (Figure 12)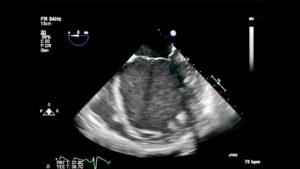
Figure 12– ME4C view of a patient with a dilated cardiomyopathy. The mitral annulus is dilated and the MV leaflets barely coapt.
In patients with HCM, abnormal papillary muscles create the substrate for LVOTO and MR. In HOCM, there can be papillary muscle hypertrophy and displacement (towards the interventricular septum) that results in LVOTO. (Figure 13, Video 7) In addition, blood flow abnormalities push the anterior leaflet into the LVOT during systole (referred to as SAM or systolic anterior motion of the mitral valve) which also causes LVOTO and a posteriorly directed jet of eccentric MR.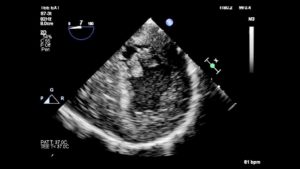
Figure 13– TG Midpapillary SAX view demonstrating a very large bifid postero-medial papillary muscle. During probe withdrawal, the mitral apparatus was seen to encroach on the LVOT and flow acceleration originated at the point of encroachment.
Video 7– TG basal SAX shows the MV leaflets encroaching on the area of the LVOT followed by a TG Midpapillary SAX view of the LV shows a markedly enlarged bifid papillary muscle that is displaced into the area of the LVOT
ABBREVIATIONS:
TG- transgastric, SAX-short axis, MV-mitral valve, ME 4C- mid-esophageal 4-chamber, CFD- color flow Doppler, ME 2C- mid-esophageal 2-chamber, M3 5C-mid-esophageal 5-chamber, TG 2C- transgastric 2-chamber, ME AV LAX- mid-esophageal aortic valve long-axis
Table 1
| Anatomic Component | What information? |
|---|---|
| Mitral annulus |
|
| Mitral leaflet pliability |
|
| Leaflet morphology |
|
| Subvalvar apparatus |
|
| Left ventricular size and compliance |
|
| Left atrium (LA) |
|
| Pulmonary veins |
|
| Presence of endocardial fibroelastosis |
|
| Aortic outlet and aortic size, presence of coarctation |
|
| Presence of associated lesions such as ASD or aortic insufficiency (AI) |
|
| Left atrial appendage |
|
Legend: AP- antero-posterior, LV- left ventricle, MR- mitral regurgitation, MS- mitral stenosis, LVEDP- left ventricular end diastolic pressure, ASD- atrial septal defect
References:
1. Alghamadi AA, Yadava M, Van Arsdell GS. Surgical management of congenital mitral stenosis. Operative Techniques in Thoracic and Cardiovascular Surgery 2010; 15 (4): 273-281
2. Kouchoukos NT, Blackstone EH, Hanley FL et al. (2013). Congenital Mitral Valve Disease Kirklin/Barratt-Boyes cardiac surgery: morphology, diagnostic criteria, natural history, techniques, results, and indications (4th ed, Vol. 2, VII, Ch. 50, pp. 1813-1828) Philadelphia, PA: Elsevier Saunders
3. Ruckman RN, Van Praagh R. Anatomic types of congenital mitral stenosis: report of 49 autopsy cases with consideration of diagnosis and surgical implications. Am J Cardiol 1978; 42:592-601
4. Verma S, Mesana TG. Mitral valve repair for mitral valve prolapse. New England Journal of Medicine 2009; 361 (23): 2261-2269
5. Freedom RM, Shi-Joon Y, Coles, JG. (2004). Congenital abnormalities of the mitral valve. In R. Freedom, Y. Shi-Joon, H. Mikailian, W. Williams (Eds.) The natural and modified history of congenital heart disease (Ch. 12, pp. 99-106) Elmhurst, NY: Blackwell Inc/Futura Division