Author: Melissa Colizza - Stollery Children’s Hospital - Edmonton, Canada
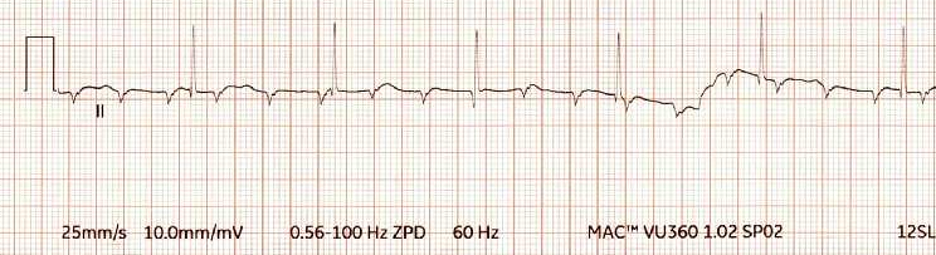
A 9-month-old male is undergoing an intracardiac repair of a complex congenital heart defect with cardiopulmonary bypass. Following release of the aortic cross clamp and rewarming, the rhythm demonstrated below is noted on the monitor. Temporary epicardial pacing wires are placed to facilitate weaning from bypass. Which of the following pacemaker modes is MOST appropriate for the rhythm demonstrated below?
EXPLANATION
Persistent post-operative atrio-ventricular (AV) block is not an uncommon complication after congenital cardiac surgery, with an incidence of 0.3-3%. Several types of congenital heart defects, such as left ventricular outflow tract (LVOT) obstruction, L-transposition of the great arteries, and ventricular septal defect (VSD), increase the risk of post-operative AV block. Most of the time, post-operative AV block is transient with a recovery rate of 81% at seven days but may require temporary epicardial pacing for optimal hemodynamics. Temporary pacing may be accomplished via transvenous, transesophageal, transcutaneous or epicardial routes. Temporary epicardial pacing is used frequently after cardiac surgery, as temporary transvenous wires are more likely to get dislodged. Furthermore, transesophageal and transcutaneous pacing requires high voltages and stimulates large portions of the myocardium simultaneously. Anesthesiologists are commonly required to set and troubleshoot temporary epicardial pacemakers in the operating room.
In the context of high-grade or complete AV block, as demonstrated in the stem, there is a lack of reliable AV conduction. At a minimum, pediatric patients require an appropriate ventricular rate for their age. AAI is a mode in which an electrical current will be delivered to the atrium at a set rate if no intrinsic atrial depolarization/voltage is sensed. However, there is no ventricular sensing or pacing involved, and ventricular contraction is dependent on intact AV conduction. Therefore, AAI is not an appropriate mode for complete heart block. VVI mode is an acceptable choice in the presence of a single ventricular lead or dysfunctional atrial wire if both ventricular and atrial wires are present. In VVI mode, an electrical current will be delivered to the ventricle at a set rate when no ventricular depolarization is sensed. In VVI mode, the absence of coordinated atrial and ventricular contraction can significantly reduce cardiac output (CO). VVI mode does not maximize cardiac output, particularly in the subset of patients who are reliant upon an “atrial kick”. DDD mode can be used in patients with both atrial and ventricular leads and allows for AV synchrony in patients with various types of AV block.
Setting the pacemaker properly requires a series of steps. Once the appropriate mode and heart rate are set, lead capture and sensitivity thresholds should be tested. Capture threshold is the minimum current in milliamps (mA) required to induce myocardial depolarization of the paced chamber. After setting the pacemaker rate above the patient’s intrinsic rate, capture threshold is found by decreasing the pacemaker output until the P wave (atrial lead) or the R wave (ventricular lead) no longer follows each pacing spike. For safety, the output is set at twice the capture threshold to a maximum of 10 mA.
The lead electrodes do not only pace, but they also sense the electrical activity of the myocardial surface. Sensitivity threshold is the minimum myocardial voltage that is detected as a P wave or R wave in millivolts (mV) by the pacemaker. If the voltage of a P wave or R wave is below the sensitivity threshold, it will not be sensed, and the pacemaker will pace at the set rate. The lower the sensitivity threshold that is set in mV, the higher is the sensitivity of the lead electrode. To determine the sensitivity threshold, the pacing rate must be temporarily set lower than patient’s native rate. The sensitivity of the lead for each appropriate chamber then must be reduced by increasing the voltage (mV) until the pacemaker no longer senses any endogenous electrical activity of the myocardium and will start to pace asynchronously. Thus, a pacing spike will appear before each P wave or R wave/QRS complex. For safety reasons, the pacemaker sensitivity should be carefully increased by decreasing the mV to about half the threshold. The general range of sensitivity for a normal pacemaker box is 0.4 to 10 mV for the atria and 0.8 to 20 mV for the ventricles. The default settings for the Medtronic pacemaker box are 0.5 mV for the atria and 2 mV for the ventricles.
Other relevant pacemaker parameters include AV delay and post-ventricular atrial refractory period (PVARP). AV delay allows time for ventricular filling after atrial contraction and before ventricular contraction. If the patient’s AV node intrinsically conducts more rapidly than the set AV delay, an endogenous ventricular beat may occur but if it does not, the pacemaker can pace the ventricle when set appropriately. AV interval is automatically determined with the pacemaker set rate but may be manually changed and allow for better ventricular filling, especially in the context of rapid atrial rate or diastolic dysfunction. Of note, an intrinsic ventricular beat will always provide better stroke volume and cardiac output as ventricular depolarization occurs in a more synchronized fashion versus depolarization from a right ventricular epicardial lead. Normal AV delay values range between 100 to 150 milliseconds (ms).
PVARP refers to the period after which the ventricular lead delivers an electrical impulse and during which the atrial lead will not be able to sense any myocardial voltage in the atria. The goal is to prevent the atrial lead from either far-field sensing of ventricular voltage or sensing atrial voltage from retrograde depolarization via a re-entry pathway. If either are sensed by the atrial lead and therefore interpreted as atrial depolarization, the ventricular lead will then pace the ventricle and induce pacemaker-mediated tachycardia. Pacemaker-mediated tachycardia presents with a ventricular paced rate that is faster than the set pacer rate and may be terminated by prolonging the PVARP.
The rhythm tracing in the stem demonstrates complete heart block. The most appropriate pacemaker mode is DDD to allow AV synchrony with an appropriately set heart rate. AAI mode is not appropriate as there is lack of AV nodal conduction. Although VVI may be useful to increase the heart rate, it is less optimal than DDD mode as it does not provide AV synchrony.
REFERENCES
Robinson JA, Leclair G, Escudero CA. Pacing in Pediatric Patients with Postoperative Atrioventricular Block. Card Electrophysiol Clin. 2023;15(4):401-411. doi: 10.1016/j.ccep.2023.06.008
Reade MC. Temporary epicardial pacing after cardiac surgery: a practical review: part 1: general considerations in the management of epicardial pacing [published correction appears in Anaesthesia. 2007 Jun;62(6):644]. Anaesthesia. 2007;62(3):264-271. doi: 10.1111/j.1365-2044.2007.04950.x
Reade MC. Temporary epicardial pacing after cardiac surgery: a practical review. Part 2: Selection of epicardial pacing modes and troubleshooting. Anaesthesia. 2007;62(4):364-373. doi: 10.1111/j.1365-2044.2007.04951.x
Andropoulos DB. Anesthesia for Congenital Heart Disease. 2nd ed. Wiley-Blackwell; 2010. Chapter 22.
