Authors: J. O’Doherty, MB, ChB AND M. Gangadharan, MD, FAAP, FASA - Children’s Memorial Hermann Hospital, University of Texas Health Science Center, Houston, TX
A full-term newborn girl is diagnosed with truncus arteriosus. A transthoracic echocardiogram reveals a main pulmonary trunk arising from the truncus arteriosus, which divides into right and left pulmonary arteries. According to the Collett and Edwards classification, which type of truncus arteriosus does this child have?
EXPLANATION
Truncus arteriosus (TA) is a cyanotic congenital cardiac abnormality characterized by a single arterial trunk supplying the aorta, pulmonary arteries, and coronary arteries. This rare condition represents one to three percent of all cardiac defects. In almost all cases, a large ventricular septal defect is present in the infundibular septum, along with a single truncal valve that is most commonly tri-leaflet.
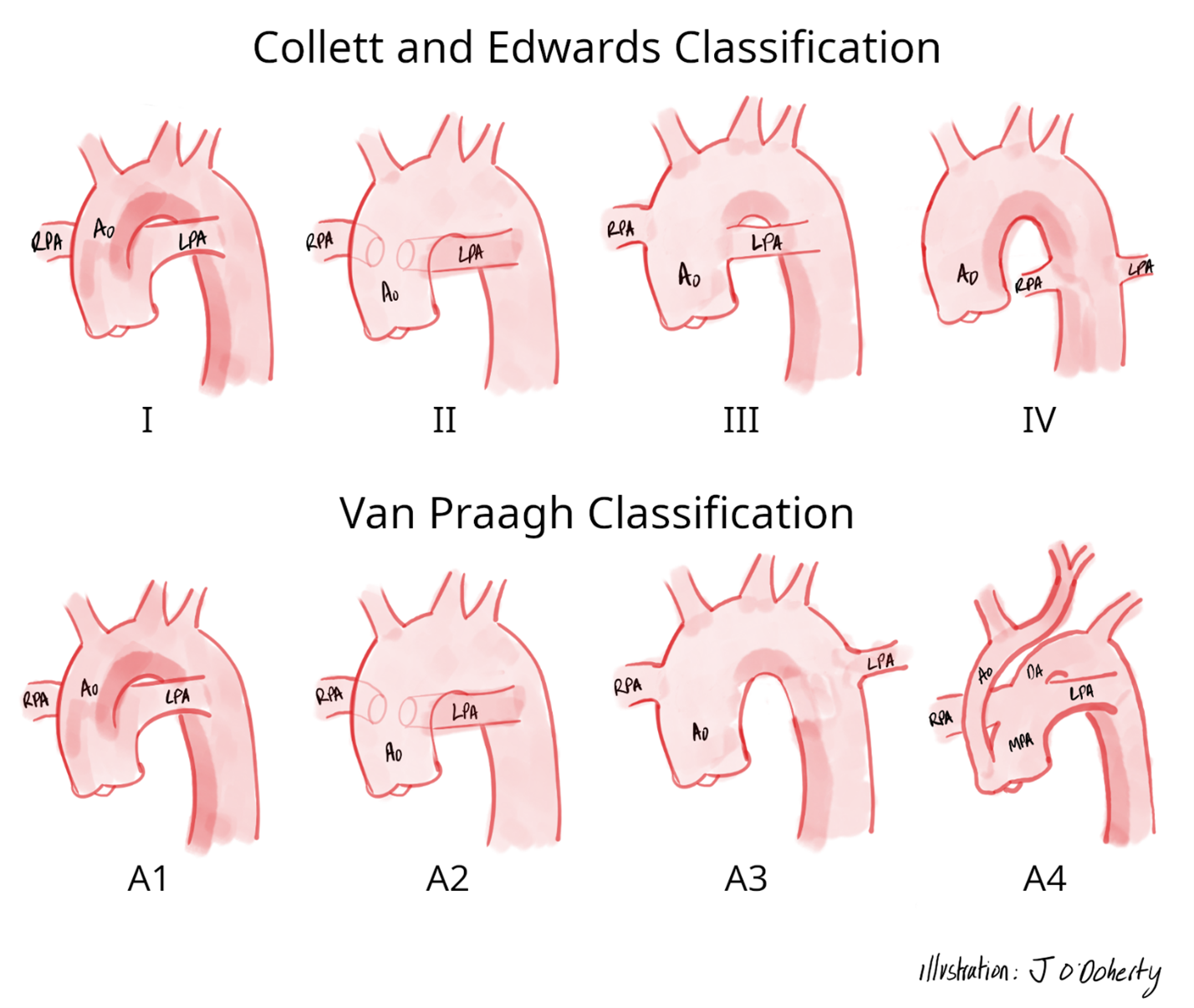
The Collett and Edwards classification (see Figure 1) categorizes TA into four types based on the pattern of origin of the pulmonary arteries, which are as follows:
• Type I is characterized by a main pulmonary artery originating from the truncus, which then divides into the left and right branch pulmonary arteries.
• Type II is characterized by an absent main pulmonary artery segment. Instead, the right and left pulmonary arteries arise separately from the posterior aspect of the truncus with their origins close to each other.
• Type III is identical to Type II, except with widely separated origins of the pulmonary arteries.
• Type IV is now recognized as a distinct clinical diagnosis, pulmonary atresia with major aortopulmonary collaterals, and is no longer considered to be a form of TA.
The classification system proposed by Van Praagh and Van Praagh (see Figure 1) divides TA into four types, including the following:
• Type A1, which is identical to Collett and Edwards Type I TA.
• Type A2, which mirrors Collett and Edwards Type II and III TA.
• Type A3 is characterized by one pulmonary artery arising from the truncus, while a second pulmonary artery is supplied by the ductus arteriosus or originates from a systemic artery.
• Type A4 includes cases with aortic arch abnormalities including hypoplasia, coarctation, and/or interruption.
Figure 1: Classification Systems of Truncus Arteriosus
It is also important to characterize the cardiac anatomy of these patients, including the anatomy and function of the truncal valve and the arrangement of the coronary arteries, as these features can significantly impact surgical outcomes. The truncal valve may be normal, regurgitant, or, rarely, stenotic. The truncal valve is tri-leaflet in most cases of TA, but can also be quadricuspid, bicuspid, pentacuspid, or unicomissural, in order of decreasing frequency. Truncal valves with moderate to severe regurgitation are usually repaired at the time of complete surgical correction.
In most cases, the right and left coronary arteries arise from their respective ostia on the right anterolateral and left posterolateral surface of the truncus. However, coronary artery anomalies are frequently associated with TA. Approximately 27% of patients have a left dominant system. The left anterior descending artery is often small, and the right coronary artery conal branch is more prominent, with large branches supplying the right ventricular outflow tract. This is important because the repair often involves the placement of a valved conduit from the right ventricle to the pulmonary artery.
Variations in coronary anatomy can significantly impact surgical outcomes. In a recent single-center, retrospective review, 34 of 107 patients undergoing truncus arteriosus repair had at least one coronary lesion, which was defined as either a single coronary, ostial stenosis, intramural coronary course or juxta commissural origin of coronaries. Patients with one or two coronary lesions had similar 5-year survival, 80% and 83%, respectively. However, patients with three coronary lesions had a 5-year survival of 24% (p = 0.003). Furthermore, there was a trend towards improved 5-year survival if interventions were performed to treat coronary artery abnormalities.
Genetic syndromes associated with truncus arteriosus are another consideration. A significant proportion of patients, 30 to 40 %, will have Chromosome 22q11.2 Deletion syndrome. These patients are 2.4 times more likely to have an aortic arch anomaly than those without this syndrome. Right aortic arch with mirror-image branching represents another common finding, occurring in 21 to 36 % of patients with TA. The ductus arteriosus is absent in 50% of patients with TA. However, the ductus arteriosus is typically present in patients with TA and a hypoplastic aortic arch anomaly.
The patient described in the vignette has a main pulmonary artery trunk arising from the truncus which divides into right and left pulmonary arteries, which is classified as Collett and Edwards Type I truncus arteriosus.
REFERENCES
Parikh R, Eisses M, Latham GJ, Joffe DC, Ross FJ. Perioperative and Anesthetic Considerations in Truncus Arteriosus. Semin Cardiothorac Vasc Anesth. 2018;22(3):285-293. doi:10.1177/1089253218778826
Menon SC, Cabalka AK, Dearani JA. Truncus Arteriosus. In: Shaddy RE, Penny DJ, Cetta F, Feltes TF, Mital S, eds. Moss and Adams’ Heart Disease in infants, children and adolescents including the fetus and young adult. 10th Edition. Philadephia, PA. Wolters Kluwer; 2022: 1009-1019
Bonilla-Ramirez C, Ibarra C, Binsalamah ZM, et al. Coronary Artery Anomalies Are Associated With Increased Mortality After Truncus Arteriosus Repair. Ann Thorac Surg. 2021;112(6):2005-2011. doi:10.1016/j.athoracsur.2020.08.082
