Author: Melissa Colizza, MD - Stollery Children’s Hospital - Edmonton AB, Canada
A one-day-old girl without prenatal care has been admitted to NICU due to cyanosis. Pulse oximetry demonstrates a SpO2 of 80% on the right hand and 89% on the left foot. Which of the following diagnoses is MOST likely in this patient?
EXPLANATION
Cyanosis in the newborn child is a rather common finding with a broad differential diagnosis. Possible causes include simple acrocyanosis, pulmonary disorders such as respiratory distress syndrome or meconium aspiration, persistent pulmonary hypertension of the newborn (PPHN), distributive shock with poor peripheral perfusion, and cyanotic congenital heart disease (CHD). All newborns are routinely screened for critical congenital heart disease using two-site pulse oximetry, which includes both a pre-ductal (right hand) and a post-ductal (foot) SpO2 reading. According to the CDC guidelines established in 2011, a SpO2 < 90%, SpO2 < 95% on three separate measurements, or a SpO2 difference of >3% between right hand and a foot warrant more extensive investigation.
A pre-ductal saturation that is more than 3% or higher than the post-ductal saturation is known as “differential cyanosis”. This can be seen in two common scenarios in which there is ductal-dependent systemic blood flow. The first scenario involves ductal dependent systemic blood flow along with some degree of antegrade blood flow through the aortic valve, such as in severe coarctation of the aorta, interrupted aortic arch, or hypoplastic left heart syndrome. In these conditions, lower body perfusion is due to the shunting of pulmonary arterial blood into the descending aorta via the patent ductus arteriosus (PDA). The second scenario occurs as a result of suprasystemic pulmonary arterial pressure or high pulmonary vascular resistance producing right-to-left shunting of blood from the pulmonary artery to the descending aorta via the PDA.
“Reverse differential cyanosis” (RDC) is defined as a pre-ductal SpO2 that is lower than the post-ductal SpO2. This occurs in situations when some quantity of oxygenated blood from the pulmonary venous return enters the descending aorta via the PDA, while deoxygenated blood is simultaneously perfusing the upper body via the pre-ductal vessels. Classically, RDC is seen in the setting of D-transposition of the great arteries (D-TGA) with one or more of the following: (1) severe pulmonary hypertension; (2) pre-ductal coarctation of the aorta, or (3) interrupted aortic arch (IAA). In D-TGA, the right ventricle ejects deoxygenated blood into the aorta, whereas oxygenated blood exits the left ventricle into the pulmonary artery. In the setting of severe pulmonary hypertension, preductal coarctation of the aorta, or interrupted aortic arch, oxygenated blood preferentially enters the descending aorta via the PDA, while the head and neck branches of the aortic arch are perfused with deoxygenated blood from the right ventricle, leading to RDC. (Fig. 1)
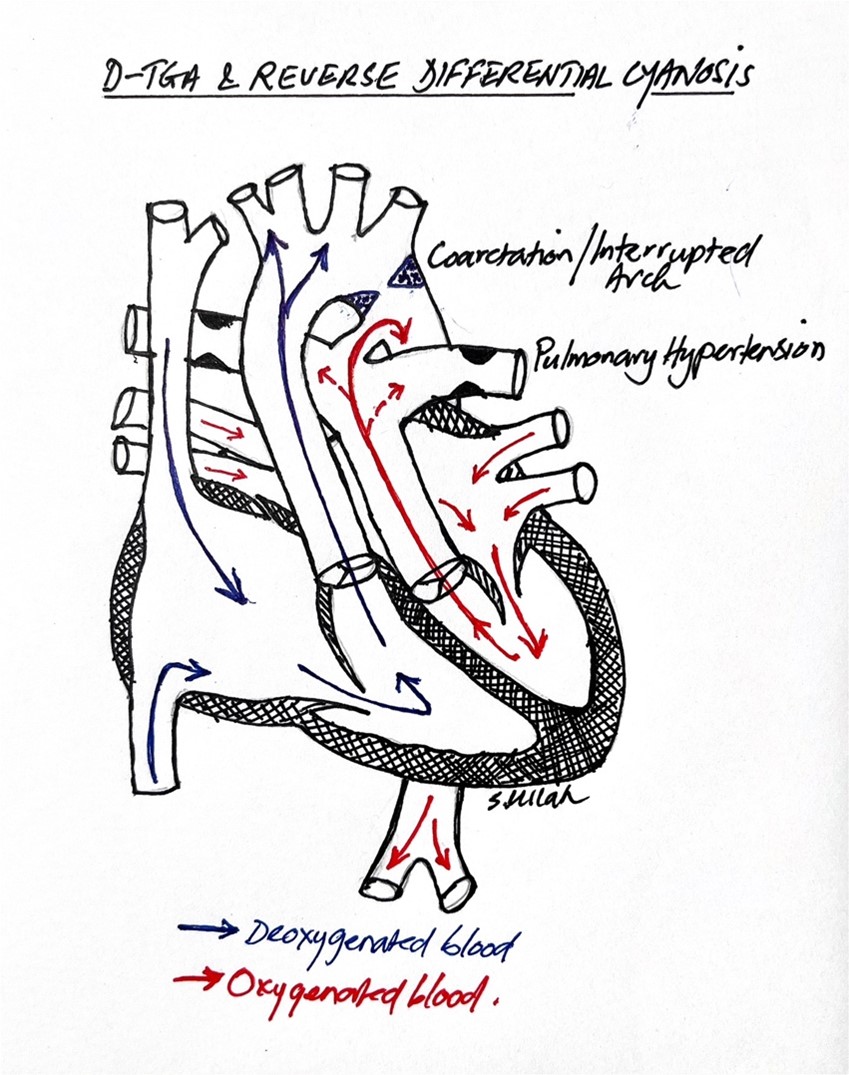 Fig.1. In the setting of D-TGA with coarctation, interrupted aortic arch or severe pulmonary hypertension, oxygenated blood preferentially streams from the PDA to the descending aorta, leading to reverse differential cyanosis.
Fig.1. In the setting of D-TGA with coarctation, interrupted aortic arch or severe pulmonary hypertension, oxygenated blood preferentially streams from the PDA to the descending aorta, leading to reverse differential cyanosis.
Reverse differential cyanosis has also been described in patients with total anomalous pulmonary venous connection (TAPVC). In supracardiac TAPVC, the persistence of two fetal circulatory shunts, the PDA and patent foramen ovale (PFO), allows for differential streaming of blood. Blood streams from the superior vena cava (SVC), which has a higher oxygen saturation due to mixing of systemic venous return with pulmonary venous blood, into the right ventricle (RV), while blood from the inferior vena cava (IVC) streams into the left ventricle (LV) via the PFO. A 2008 case report by Yap et al describes a newborn with RDC in the context of supracardiac TAPVC. In this patient, the left pulmonary veins drained into the innominate vein via a vertical vein, while the right pulmonary veins entered the SVC directly. A transthoracic echocardiogram demonstrated that the SVC blood return with a higher oxygen saturation preferentially entered the RV before streaming into the descending aorta via the PDA. The blood returning from the IVC with lower oxygen saturation preferentially streamed into the left atrium through a moderate ASD, then into the LV, and finally to the aorta. Thus, the ascending aorta and aortic arch were supplied by relatively deoxygenated blood (Figure 2). Chow et al also published a case series of two neonates with obstructed TAPVC presenting with RDC. Another lesion which may cause RDC is an anomalous right subclavian artery that is connected by the ductus to the right pulmonary artery. Conversely, a newborn with infracardiac TAPVC could present with differential cyanosis due to IVC return with a higher oxygen saturation streaming into the LV via the PFO and then entering the aorta and head/neck vessels.
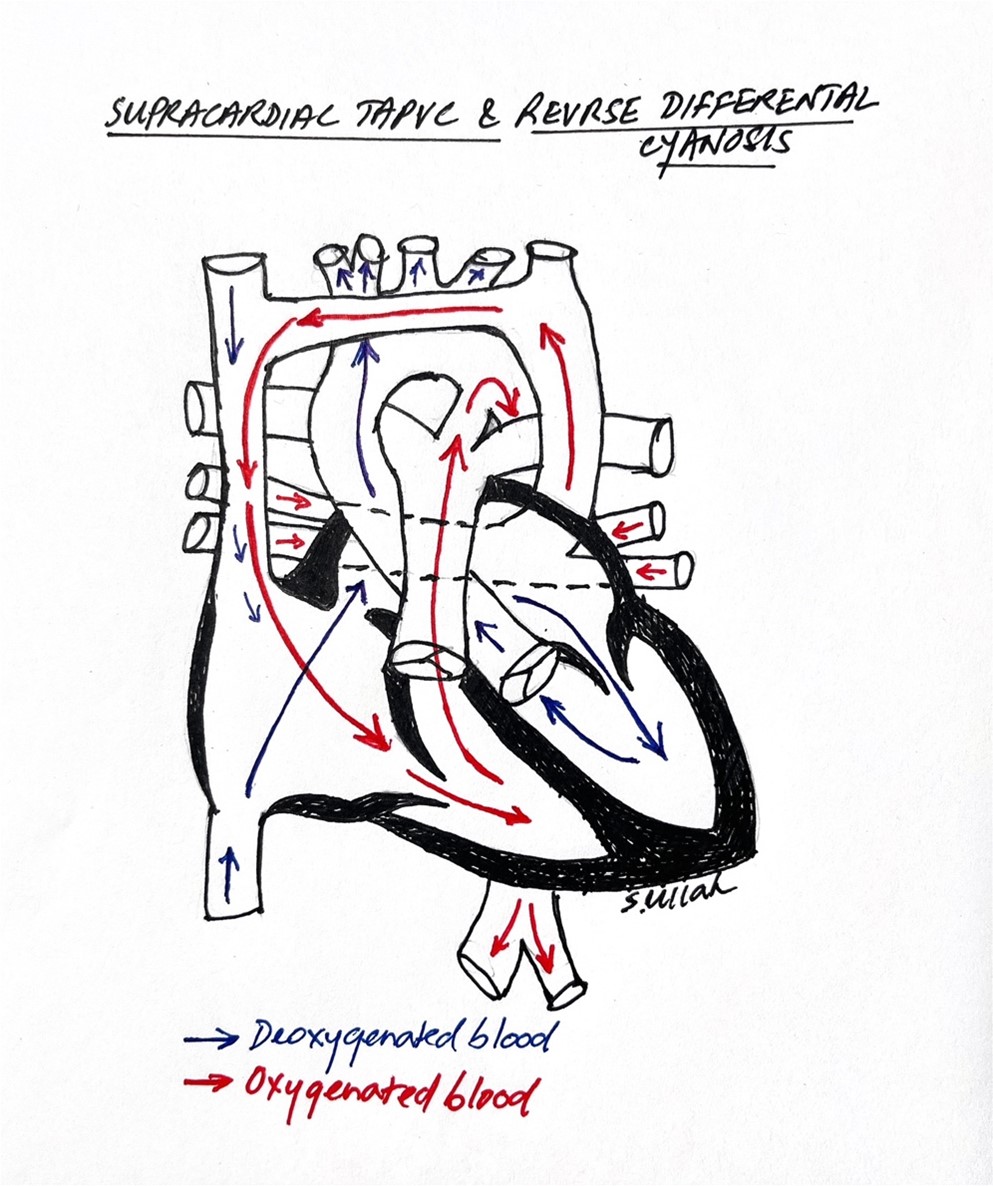 Fig.2. In supracardiac TAPVC, SVC blood return of higher oxygen saturation streams into the RV and then across the PDA into the descending aorta, while the deoxygenated IVC return streams across the PFO to the LV and is ejected into the upper body, producing reverse differential cyanosis.
Fig.2. In supracardiac TAPVC, SVC blood return of higher oxygen saturation streams into the RV and then across the PDA into the descending aorta, while the deoxygenated IVC return streams across the PFO to the LV and is ejected into the upper body, producing reverse differential cyanosis.
The correct answer choice for the patient described in the stem presenting with RDC is TAPVC. Both Hypoplastic Left Heart Syndrome (HLHS) and PPHN would give rise to differential cyanosis as opposed to RDC and are therefore incorrect.
REFERENCES
Yap SH, Anania N, Alboliras ET, Lilien LD. Reversed differential cyanosis in the newborn: a clinical finding in the supracardiac total anomalous pulmonary venous connection. Pediatr Cardiol. 2009;30(3):359-362. doi:10.1007/s00246-008-9314-0
Chow PC, Chen RHS, Rocha BA, Yam NLH. Reversed differential cyanosis in two neonates with obstructed supracardiac total anomalous pulmonary venous drainage. Res Pediatr Neonatol. 2019; 3(5). RPN.000574. doi: 10.31031/RPN.2019.03.000574
Oster ME, Aucott SW, Glidewell J et al. Lessons Learned From Newborn Screening for Critical Congenital Heart Defects. Pediatrics. 2016 May;137(5): e20154573. doi: 10.1542/peds.2015-4573.
