Authors: Katarzyna Dlugosz Sledz, MD and M. Gangadharan, MD, FAAP, FASA - Children’s Memorial Hermann Hospital, University of Texas Health Science Center, Houston, TX AND Destiny F. Chau, MD - Arkansas Children’s Hospital/University of Arkansas for Medical Sciences, Little Rock, AR
A 20-year-old woman with a history of pulmonary hypertension treated with riociguat presents for a pulmonary hypertension study. Which of the following mechanisms BEST describes the pharmacologic action of riociguat?
EXPLANATION
Pulmonary hypertension is defined by mean pulmonary artery pressure (PAP) greater than 20 mmHg at rest in a biventricular heart and the transpulmonary gradient is > 6mmHg in a univentricular heart in pediatric patients greater than three months of age. Pulmonary arterial hypertension, also termed precapillary pulmonary hypertension, is additionally characterized by a pulmonary vascular resistance index (PVRI) greater than three wood units per m2. Referral to a pediatric pulmonary hypertension specialist is a necessity for the diagnosis and management of pediatric patients with pulmonary hypertension. Formal diagnosis is made by cardiac catheterization and acute vasoreactivity testing. Treatment consists of diuretics, anticoagulants, digoxin, supplemental oxygen, and calcium channel blockers in patients with positive acute pulmonary vasoreactivity. Patients with negative vasoreactivity testing are typically treated with the addition of one or more pulmonary vasodilators.
Three mediators, endothelin, nitric oxide, and prostacyclin, regulate pulmonary arterial vascular tone. The molecular pathways for these mediators are illustrated in the figure 1 below, along with the therapeutic targets for each. The endothelin pathway mediates vasoconstriction, whereas the prostacyclin and nitric oxide pathways mediate vasodilatation. Targeted therapies are aimed at improving the balance between vasoconstriction and vasodilation which is abnormal in pulmonary hypertension.
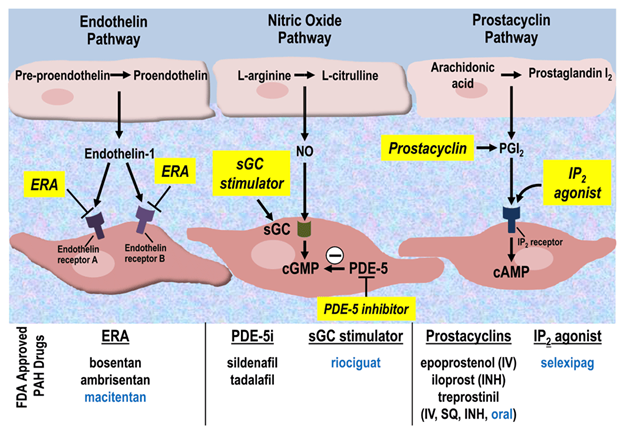 Figure 1. Molecular targets for pulmonary hypertension drug therapies. Newly approved therapies are listed in blue. cAMP, cyclic adenosine monophosphate; cGMP, cyclic guanylate monophosphate; ERA, endothelin receptor antagonist; FDA, US Food and Drug Administration; INH, inhaled; IP2, prostacyclin receptor 2; IV, intravenous; NO, nitric oxide; PAH, pulmonary arterial hypertension; PDE-5, phosphodiesterase-5; PDE-5i, phosphodiesterase-5 inhibitor; PGI2, prostaglandin I2; sGC, soluble guanylate cyclase; SQ, subcutaneous. (Tsai H, Sung YK, and de Jesus Perez V. Recent advances in the management of pulmonary arterial hypertension. F1000Research. 2016;5:2755. Creative Commons License)
Figure 1. Molecular targets for pulmonary hypertension drug therapies. Newly approved therapies are listed in blue. cAMP, cyclic adenosine monophosphate; cGMP, cyclic guanylate monophosphate; ERA, endothelin receptor antagonist; FDA, US Food and Drug Administration; INH, inhaled; IP2, prostacyclin receptor 2; IV, intravenous; NO, nitric oxide; PAH, pulmonary arterial hypertension; PDE-5, phosphodiesterase-5; PDE-5i, phosphodiesterase-5 inhibitor; PGI2, prostaglandin I2; sGC, soluble guanylate cyclase; SQ, subcutaneous. (Tsai H, Sung YK, and de Jesus Perez V. Recent advances in the management of pulmonary arterial hypertension. F1000Research. 2016;5:2755. Creative Commons License)
Prostacyclin analogs, such as iloprost, epoprostenol, and treprostinil, increase cyclic adenosine monophosphate (cAMP) levels in pulmonary vascular smooth muscle cells resulting in vasodilation. Endothelin receptor blockers, such as bosentan, ambrisentan, and macitentan prevent vasoconstriction and smooth muscle proliferation via endothelin A and B receptor blockade. The nitric oxide pathway results in increased levels of cyclic guanylate monophosphate (cGMP), which mediates pulmonary vasodilation. Exogenous administration of nitric oxide increases the production of cGMP, while phosphodiesterase inhibitors, such as sildenafil and tadalafil, reduce the breakdown of cGMP.
Riociguat is a direct soluble guanylate cyclase (sGC) stimulator that increases the sensitivity of guanylate cyclase to nitric oxide, resulting in increased intracellular cGMP levels, which in turn leads to a vasodilatory and antiproliferative effect on vascular smooth muscle. Riociguat (trade name Adempas®) has been approved by the European Medicines Agency to treat pulmonary hypertension in children older than six 6 years with systolic blood pressure greater than 90 mmHg and children older than 12 years with systolic blood pressure greater than 95mmHg. Riociguat has not received FDA approval for pediatric use in the United States. The side effects of riociguat include systemic hypotension, hemoptysis, pulmonary hemorrhage, headache, and nausea. Concomitant use of riociguat with a phosphodiesterase-5 inhibitor is contraindicated due to an exaggerated effect on blood pressure. There are case reports of the successful use of riociguat in children. Domingo and colleagues reported two cases of infants with refractory supra-systemic pulmonary hypertension who were weaned off nitric oxide after the administration of riociguat.
The correct answer is B. Riociguat is a direct soluble guanylate cyclase (sGC) stimulator that increases the sensitivity of guanylate cyclase to nitric oxide, resulting in increased levels of intracellular cGMP. Although sildenafil and tadalafil also act via the nitric oxide pathway, they produce their therapeutic effect via phosphodiesterase-5 inhibition. Bosentan and ambrisentan are endothelin receptor antagonists.
REFERENCES
Beghetti M, Gorenflo M, Ivy DD, Moledina S, Bonnet D. Treatment of pediatric pulmonary arterial hypertension: A focus on the NO-sGC-cGMP pathway. Pediatr Pulmonol. 2019;54(10):1516-1526. doi:10.1002/ppul.24442
https://www.ema.europa.eu/en/documents/product-information/adempas-epar-product-information_en.pdf Accessed on 9.7.2024
Domingo LT, Ivy DD, Abman SH, et al. Novel use of riociguat in infants with severe pulmonary arterial hypertension unable to wean from inhaled nitric oxide. Front Pediatr. 2022; 10:1014922. doi:10.3389/fped.2022.1014922
Hansmann G, Koestenberger M, Alastalo TP, et al. 2019 updated consensus statement on the diagnosis and treatment of pediatric pulmonary hypertension: The European Pediatric Pulmonary Vascular Disease Network (EPPVDN), endorsed by AEPC, ESPR, and ISHLT. J Heart Lung Transplant. 2019;38(9):879-901. doi:10.1016/j.healun.2019.06.022
Jone PN, Ivy DD, Hauck A, et al. Pulmonary Hypertension in Congenital Heart Disease: A Scientific Statement From the American Heart Association. Circ Heart Fail. 2023;16(7):e00080. doi:10.1161/HHF.0000000000000080
